Shelterin Telomere Protection Protein 1 Reduction Causes Telomere Attrition and Cellular Senescence via Sirtuin 1 Deacetylase in Chronic Obstructive Pulmonary Disease
- PMID: 27559927
- PMCID: PMC5248966
- DOI: 10.1165/rcmb.2016-0198OC
Shelterin Telomere Protection Protein 1 Reduction Causes Telomere Attrition and Cellular Senescence via Sirtuin 1 Deacetylase in Chronic Obstructive Pulmonary Disease
Abstract
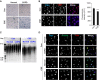
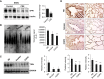
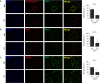
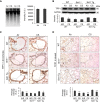
Lung cellular senescence and inflammatory response are the key events in the pathogenesis of chronic obstructive pulmonary disease (COPD) when cigarette smoke (CS) is the main etiological factor. Telomere dysfunction is induced by either critical shortening or disruption of the shelterin complex, leading to cellular senescence. However, it remains unknown whether disruption of the shelterin complex is responsible for CS-induced lung cellular senescence. Here we show that telomere protection protein 1 (TPP1) levels are reduced on telomeres in lungs from mice with emphysema, as well as in lungs from smokers and from patients with COPD. This is associated with persistent telomeric DNA damage, leading to cellular senescence. CS disrupts the interaction of TPP1 with the Sirtuin 1 (Sirt1) complex, leading to increased TPP1 acetylation and degradation. Lung fibroblasts deficient in Sirt1 or treated with a selective Sirt1 inhibitor exhibit increased cellular senescence and decreased TPP1 levels, whereas Sirt1 overexpression and pharmacological activation protect against CS-induced TPP1 reduction and telomeric DNA damage. Our findings support an essential role of TPP1 in protecting CS-induced telomeric DNA damage and cellular senescence, and therefore provide a rationale for a potential therapy for COPD, on the basis of the shelterin complex, in attenuating cellular senescence.
Keywords: Sirtuin1; cellular senescence; emphysema; shelterin complex; telomeric DNA damage.
Figures



References
-
- Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, Reddel RR. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol. 2009;16:1244–1251. - PubMed
-
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

