The characteristics and clinical outcome of drug-induced liver injury in a Chinese hospital: A retrospective cohort study
- PMID: 27559976
- PMCID: PMC5400343
- DOI: 10.1097/MD.0000000000004683
The characteristics and clinical outcome of drug-induced liver injury in a Chinese hospital: A retrospective cohort study
Abstract
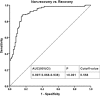
The aim of this cohort study was to determine the characteristics and clinical outcome of 287 patients with drug-induced liver injury (DILI) in a Chinese hospital.Between January 2008 and January 2013, individuals who were diagnosed with DILI were selected. The complete medical records of each case were reviewed, and factors for the outcome of patients with DILI were extracted and analyzed using univariate and multivariate analysis.Two hundred eighty-seven cases identified as DILI were included in the study. A total of 105 different drugs were considered to be related to the hepatotoxicity. The main causative group of drugs was Chinese herb (n = 111). Liver failure developed in 9 (3.1%) patients, and 2 died (0.7%). Overall, complete recovery occurred in 92 (32.1%) patients. Univariate analysis and binary logistic regression analysis identified the digestive symptoms, jaundice, total bilirubin (TBIL), and direct bilirubin (DBIL) as independent factors for the non-recovery of DILI. Then the prediction model, including digestive symptoms, jaundice, TBIL, and DBIL, was built by using binary logistic regression analysis again. Receiver operating characteristic curve validated the strong power (area under the curve (AUC) = 0.907) of prediction model for predicting the DILI non-recovery.DILI is an important cause of liver test abnormalities, and Chinese herb represented the most common drug group. The factors such as digestive symptoms, jaundice, TBIL, and DBIL have effect on DILI outcomes. The prediction model, including digestive symptoms, jaundice, TBIL, and DBIL, established in this study is really an excellent predictive tool for non-recovery of DILI patients.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




References
-
- Andres E. Drug-induced hepatotoxicity. N Engl J Med 2003; 349:1974–1976. - PubMed
-
- Grant LM, Rockey DC. Drug-induced liver injury. Curr Opin Gastroenterol 2012; 28:198–202. - PubMed
-
- Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis 2014; 34:134–144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

