Fucosylation of LAMP-1 and LAMP-2 by FUT1 correlates with lysosomal positioning and autophagic flux of breast cancer cells
- PMID: 27560716
- PMCID: PMC5108328
- DOI: 10.1038/cddis.2016.243
Fucosylation of LAMP-1 and LAMP-2 by FUT1 correlates with lysosomal positioning and autophagic flux of breast cancer cells
Abstract
Alpha1,2-fucosyltransferases, FUT1 and FUT2, which transfer fucoses onto the terminal galactose of N-acetyl-lactosamine via α1,2-linkage have been shown to be highly expressed in various types of cancers. A few studies have shown the involvement of FUT1 substrates in tumor cell proliferation and migration. Lysosome-associated membrane protein 1, LAMP-1, has been reported to carry alpha1,2-fucosylated Lewis Y (LeY) antigens in breast cancer cells, however, the biological functions of LeY on LAMP-1 remain largely unknown. Whether or not its family member, LAMP-2, displays similar modifications and functions as LAMP-1 has not yet been addressed. In this study, we have presented evidence supporting that both LAMP-1 and 2 are substrates for FUT1, but not FUT2. We have also demonstrated the presence of H2 and LeY antigens on LAMP-1 by a targeted nanoLC-MS(3) and the decreased levels of fucosylation on LAMP-2 by MALDI-TOF analysis upon FUT1 knockdown. In addition, we found that the expression of LeY was substantial in less invasive ER+/PR+/HER- breast cancer cells (MCF-7 and T47D) but negligible in highly invasive triple-negative MDA-MB-231 cells, of which LeY levels were correlated with the levels of LeY carried by LAMP-1 and 2. Intriguingly, we also observed a striking change in the subcellular localization of lysosomes upon FUT1 knockdown from peripheral distribution of LAMP-1 and 2 to a preferential perinuclear accumulation. Besides that, knockdown of FUT1 led to an increased rate of autophagic flux along with diminished activity of mammalian target of rapamycin complex 1 (mTORC1) and enhanced autophagosome-lysosome fusion. This may be associated with the predominantly perinuclear distribution of lysosomes mediated by FUT1 knockdown as lysosomal positioning has been reported to regulate mTOR activity and autophagy. Taken together, our results suggest that downregulation of FUT1, which leads to the perinuclear localization of LAMP-1 and 2, is correlated with increased rate of autophagic flux by decreasing mTOR signaling and increasing autolysosome formation.
Figures




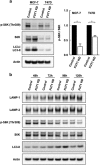
References
-
- Goupille C, Marionneau S, Bureau V, Hallouin F, Meichenin M, Rocher J et al. Alpha1,2-fucosyltransferase increases resistance to apoptosis of rat colon carcinoma cells. Glycobiology 2000; 10: 375–382. - PubMed
-
- Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoen N, Clement M et al. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 2001; 83: 565–573. - PubMed
-
- Oriol R, Mollicone R, Coullin P, Dalix AM, Candelier JJ. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl 1992; 27: 28–38. - PubMed
-
- Aubert M, Panicot L, Crotte C, Gibier P, Lombardo D, Sadoulet MO et al. Restoration of alpha(1,2) fucosyltransferase activity decreases adhesive and metastatic properties of human pancreatic cancer cells. Cancer Res 2000; 60: 1449–1456. - PubMed
-
- Labarriere N, Piau JP, Otry C, Denis M, Lustenberger P, Meflah K et al. H blood group antigen carried by CD44V modulates tumorigenicity of rat colon carcinoma cells. Cancer Res 1994; 54: 6275–6281. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

