Novel NAD+-Farnesal Dehydrogenase from Polygonum minus Leaves. Purification and Characterization of Enzyme in Juvenile Hormone III Biosynthetic Pathway in Plant
- PMID: 27560927
- PMCID: PMC4999093
- DOI: 10.1371/journal.pone.0161707
Novel NAD+-Farnesal Dehydrogenase from Polygonum minus Leaves. Purification and Characterization of Enzyme in Juvenile Hormone III Biosynthetic Pathway in Plant
Abstract
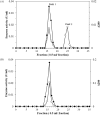
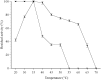
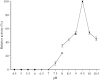
Juvenile Hormone III is of great concern due to negative effects on major developmental and reproductive maturation in insect pests. Thus, the elucidation of enzymes involved JH III biosynthetic pathway has become increasing important in recent years. One of the enzymes in the JH III biosynthetic pathway that remains to be isolated and characterized is farnesal dehydrogenase, an enzyme responsible to catalyze the oxidation of farnesal into farnesoic acid. A novel NAD+-farnesal dehydrogenase of Polygonum minus was purified (315-fold) to apparent homogeneity in five chromatographic steps. The purification procedures included Gigacap S-Toyopearl 650M, Gigacap Q-Toyopearl 650M, and AF-Blue Toyopearl 650ML, followed by TSK Gel G3000SW chromatographies. The enzyme, with isoelectric point of 6.6 is a monomeric enzyme with a molecular mass of 70 kDa. The enzyme was relatively active at 40°C, but was rapidly inactivated above 45°C. The optimal temperature and pH of the enzyme were found to be 35°C and 9.5, respectively. The enzyme activity was inhibited by sulfhydryl agent, chelating agent, and metal ion. The enzyme was highly specific for farnesal and NAD+. Other terpene aldehydes such as trans- cinnamaldehyde, citral and α- methyl cinnamaldehyde were also oxidized but in lower activity. The Km values for farnesal, citral, trans- cinnamaldehyde, α- methyl cinnamaldehyde and NAD+ were 0.13, 0.69, 0.86, 1.28 and 0.31 mM, respectively. The putative P. minus farnesal dehydrogenase that's highly specific towards farnesal but not to aliphatic aldehydes substrates suggested that the enzyme is significantly different from other aldehyde dehydrogenases that have been reported. The MALDI-TOF/TOF-MS/MS spectrometry further identified two peptides that share similarity to those of previously reported aldehyde dehydrogenases. In conclusion, the P. minus farnesal dehydrogenase may represent a novel plant farnesal dehydrogenase that exhibits distinctive substrate specificity towards farnesal. Thus, it was suggested that this novel enzyme may be functioning specifically to oxidize farnesal in the later steps of JH III pathway. This report provides a basic understanding for recombinant production of this particular enzyme. Other strategies such as adding His-tag to the protein makes easy the purification of the protein which is completely different to the native protein. Complete sequence, structure and functional analysis of the enzyme will be important for developing insect-resistant crop plants by deployment of transgenic plant.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Purification and Characterization of a Novel NAD(P)+-Farnesol Dehydrogenase from Polygonum minus Leaves.PLoS One. 2015 Nov 23;10(11):e0143310. doi: 10.1371/journal.pone.0143310. eCollection 2015. PLoS One. 2015. PMID: 26600471 Free PMC article.
-
Farnesol and farnesal dehydrogenase(s) in corpora allata of the tobacco hornworm moth, Manduca sexta.J Lipid Res. 1983 Dec;24(12):1586-94. J Lipid Res. 1983. PMID: 6366103
-
Aldehyde dehydrogenase 3 converts farnesal into farnesoic acid in the corpora allata of mosquitoes.Insect Biochem Mol Biol. 2013 Aug;43(8):675-82. doi: 10.1016/j.ibmb.2013.04.002. Epub 2013 Apr 29. Insect Biochem Mol Biol. 2013. PMID: 23639754 Free PMC article.
-
Monoterpene alcohol metabolism: identification, purification, and characterization of two geraniol dehydrogenase isoenzymes from Polygonum minus leaves.Biosci Biotechnol Biochem. 2012;76(8):1463-70. doi: 10.1271/bbb.120137. Epub 2012 Aug 7. Biosci Biotechnol Biochem. 2012. PMID: 22878188
-
Antiquitin, a relatively unexplored member in the superfamily of aldehyde dehydrogenases with diversified physiological functions.Cell Mol Life Sci. 2006 Dec;63(24):2881-5. doi: 10.1007/s00018-006-6089-4. Cell Mol Life Sci. 2006. PMID: 17131062 Free PMC article. Review.
Cited by
-
A virus carries a gene encoding juvenile hormone acid methyltransferase, a key regulatory enzyme in insect metamorphosis.Sci Rep. 2017 Oct 19;7(1):13522. doi: 10.1038/s41598-017-14059-8. Sci Rep. 2017. PMID: 29051595 Free PMC article.
-
Biosynthesis of geranate via isopentenol utilization pathway in Escherichia coli.Biotechnol Bioeng. 2023 Jan;120(1):230-238. doi: 10.1002/bit.28255. Epub 2022 Nov 1. Biotechnol Bioeng. 2023. PMID: 36224741 Free PMC article.
References
-
- Ishaaya I, Palli SR, Horowitz AR. Advanced technologies for managing insect pests. New York: Springer Science & Business Media; 2012.
-
- Jadhav S. Effect of Annona seed extract on nutritional index (NIG) of Corcyra cephalonica (St.). Adv Pharmacol Toxicol. 2014; 15 (2): 13–7.
-
- Furuta K. Design, synthesis and biological activity of novel anti-juvenile hormone agents. J Pestic Sci. 2010; 35 (4): 494–5.
-
- Jindra M, Bellés X, Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. 2015;11: 39–46. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

