Risk of Ischemic Stroke, Hemorrhagic Stroke, Bleeding, and Death in Patients Switching from Vitamin K Antagonist to Dabigatran after an Ablation
- PMID: 27560967
- PMCID: PMC4999147
- DOI: 10.1371/journal.pone.0161768
Risk of Ischemic Stroke, Hemorrhagic Stroke, Bleeding, and Death in Patients Switching from Vitamin K Antagonist to Dabigatran after an Ablation
Abstract
Background: Safety regarding switching from vitamin K antagonist (VKA) to dabigatran therapy in post-ablation patients has never been investigated and safety data for this is urgently needed. The objective of this study was to examine if switch from VKA to dabigatran increased the risk of stroke, bleeding, and death in patients after ablation for atrial fibrillation.
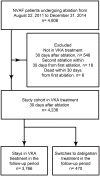
Methods: Through the Danish nationwide registries, patients with non-valvular atrial fibrillation undergoing ablation were identified, in the period between August 22nd 2011 and December 31st 2015. The risk of ischemic stroke, hemorrhagic stroke, bleeding, and death, related to switching from VKA to dabigatran was examined using a multivariable Poisson regression model, where Incidence rate ratios (IRR) were estimated using VKA as reference.
Results: In total, 4,236 patients were included in the study cohort. The minority (n = 470, 11%) switched to dabigatran in the follow up period leaving the majority (n = 3,766, 89%) in VKA treatment. The patients in the dabigatran group were older, were more often males, and had higher CHA2DS2-VASc, and HAS-BLED scores. The incident rates of bleeding and death were almost twice as high in the dabigatran group compared with the VKA group. When adjusting for the individual components included in the CHA2DS2-VASc and HAS-BLED scores, the multivariable Poisson analyses yielded a non-significant IRR (95%CI) of 1.64 (0.72-3.75) for bleeding and of 1.41 (0.66-3.00) for death associated with the dabigatran group, compared to the VKA group. A significant increased risk of bleeding was found in the 110mg bid group with an IRR (95%CI) of 4.49(1.40-14.5).
Conclusion: Shifting from VKA to dabigatran after ablation was associated with twice as high incidence of bleeding compared to the incidence in patients staying in VKA treatment. The only significant increased risk found in the adjusted analyses was for bleeding with 110mg bid dabigatran and not for 150mg bid. Since there was no dose-response for bleeding, the switch from VKA to dabigatran in itself was not a risk factor for bleeding.
Conflict of interest statement
Boehringer Ingelheim manufactures dabigatran. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study.Circulation. 2015 Sep 29;132(13):1252-60. doi: 10.1161/CIRCULATIONAHA.115.015710. Epub 2015 Jul 21. Circulation. 2015. PMID: 26199338 Free PMC article.
-
Dabigatran and vitamin K antagonists' use in naïve patients with non-valvular atrial fibrillation: a cross-sectional study of primary care-based electronic health records.Eur J Clin Pharmacol. 2017 Oct;73(10):1323-1330. doi: 10.1007/s00228-017-2305-4. Epub 2017 Jul 19. Eur J Clin Pharmacol. 2017. PMID: 28725983
-
Dabigatran and warfarin for secondary prevention of stroke in atrial fibrillation patients: a nationwide cohort study.Am J Med. 2014 Dec;127(12):1172-8.e5. doi: 10.1016/j.amjmed.2014.07.023. Epub 2014 Sep 1. Am J Med. 2014. PMID: 25193361
-
Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials.Europace. 2018 Oct 1;20(10):1612-1620. doi: 10.1093/europace/euy133. Europace. 2018. PMID: 29982383
-
Real Data on Effectiveness, Tolerability and Safety of New Oral Anticoagulant Agents: Focus on Dabigatran.High Blood Press Cardiovasc Prev. 2016 Jun;23(2):115-22. doi: 10.1007/s40292-016-0150-7. Epub 2016 May 20. High Blood Press Cardiovasc Prev. 2016. PMID: 27207360 Review.
Cited by
-
Patients with atrial fibrillation and permanent pacemaker: Temporal changes in patient characteristics and pharmacotherapy.PLoS One. 2018 Mar 28;13(3):e0195175. doi: 10.1371/journal.pone.0195175. eCollection 2018. PLoS One. 2018. PMID: 29590209 Free PMC article.
References
-
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, et al. (2012) 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14: 528–606. 10.1093/europace/eus027 - DOI - PubMed
-
- (n.d.). NBV 2015—Dansk Cardiologisk Selskab—15 Atrieflimren og atrieflagren. Available: http://nbv.cardio.dk/af. Accessed 3 February 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

