Non-invasive MRI Assessments of Tissue Microstructures and Macromolecules in the Eye upon Biomechanical or Biochemical Modulation
- PMID: 27561353
- PMCID: PMC5000015
- DOI: 10.1038/srep32080
Non-invasive MRI Assessments of Tissue Microstructures and Macromolecules in the Eye upon Biomechanical or Biochemical Modulation
Abstract
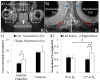
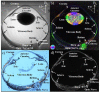
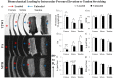
The microstructural organization and composition of the corneoscleral shell (CSS) determine the biomechanical behavior of the eye, and are important in diseases such as glaucoma and myopia. However, limited techniques can assess these properties globally, non-invasively and quantitatively. In this study, we hypothesized that multi-modal magnetic resonance imaging (MRI) can reveal the effects of biomechanical or biochemical modulation on CSS. Upon intraocular pressure (IOP) elevation, CSS appeared hyperintense in both freshly prepared ovine eyes and living rat eyes using T2-weighted MRI. Quantitatively, transverse relaxation time (T2) of CSS increased non-linearly with IOP at 0-40 mmHg and remained longer than unloaded tissues after being unpressurized. IOP loading also increased fractional anisotropy of CSS in diffusion tensor MRI without apparent change in magnetization transfer MRI, suggestive of straightening of microstructural fibers without modification of macromolecular contents. Lastly, treatments with increasing glyceraldehyde (mimicking crosslinking conditions) and chondroitinase-ABC concentrations (mimicking glycosaminoglycan depletion) decreased diffusivities and increased magnetization transfer in cornea, whereas glyceraldehyde also increased magnetization transfer in sclera. In summary, we demonstrated the changing profiles of MRI contrast mechanisms resulting from biomechanical or biochemical modulation of the eye non-invasively. Multi-modal MRI may help evaluate the pathophysiological mechanisms in CSS and the efficacy of corneoscleral treatments.
Conflict of interest statement
The author(s) have made the following disclosure(s): J.S.S.: Royalties e Zeiss, Dublin, CA (for intellectual property licensed by the Massachusetts Institute of Technology and Massachusetts Eye and Ear Infirmary).
Figures



Similar articles
-
Magic angle-enhanced MRI of fibrous microstructures in sclera and cornea with and without intraocular pressure loading.Invest Ophthalmol Vis Sci. 2014 Aug 7;55(9):5662-72. doi: 10.1167/iovs.14-14561. Invest Ophthalmol Vis Sci. 2014. PMID: 25103267 Free PMC article.
-
Visualizing dynamic alterations of vitreous viscosity during elevated intraocular pressure in glaucoma with a Near-infrared/Magnetic resonance imaging dual-modal nanoprobe.J Colloid Interface Sci. 2025 Feb;679(Pt B):529-538. doi: 10.1016/j.jcis.2024.10.110. Epub 2024 Oct 20. J Colloid Interface Sci. 2025. PMID: 39467364
-
Citicoline Modulates Glaucomatous Neurodegeneration Through Intraocular Pressure-Independent Control.Neurotherapeutics. 2021 Apr;18(2):1339-1359. doi: 10.1007/s13311-021-01033-6. Epub 2021 Apr 13. Neurotherapeutics. 2021. PMID: 33846961 Free PMC article.
-
Intraocular pressure spikes in keratectasia, axial myopia, and glaucoma.Optom Vis Sci. 2008 Oct;85(10):1018-26. doi: 10.1097/OPX.0b013e3181890e91. Optom Vis Sci. 2008. PMID: 18832981 Review.
-
Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma.Biomolecules. 2021 Feb 24;11(3):340. doi: 10.3390/biom11030340. Biomolecules. 2021. PMID: 33668357 Free PMC article. Review.
Cited by
-
In Vivo Evaluation of the Visual Pathway in Streptozotocin-Induced Diabetes by Diffusion Tensor MRI and Contrast Enhanced MRI.PLoS One. 2016 Oct 21;11(10):e0165169. doi: 10.1371/journal.pone.0165169. eCollection 2016. PLoS One. 2016. PMID: 27768755 Free PMC article.
-
Stretch-Induced Uncrimping of Equatorial Sclera Collagen Bundles.J Biomech Eng. 2023 May 1;145(5):054503. doi: 10.1115/1.4056354. J Biomech Eng. 2023. PMID: 36459150 Free PMC article.
-
Application of advanced magnetic resonance imaging in glaucoma: a narrative review.Quant Imaging Med Surg. 2022 Mar;12(3):2106-2128. doi: 10.21037/qims-21-790. Quant Imaging Med Surg. 2022. PMID: 35284278 Free PMC article. Review.
-
Age-related Changes in Eye, Brain and Visuomotor Behavior in the DBA/2J Mouse Model of Chronic Glaucoma.Sci Rep. 2018 Mar 15;8(1):4643. doi: 10.1038/s41598-018-22850-4. Sci Rep. 2018. PMID: 29545576 Free PMC article.
-
Magnetic Resonance Conditional Microinjector.J Imaging. 2018 Dec 30;5(1):4. doi: 10.3390/jimaging5010004. J Imaging. 2018. PMID: 34470181 Free PMC article.
References
-
- Liu J. & He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci 50, 2224–2229 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

