Discovery of a Carbazole-Derived Lead Drug for Human African Trypanosomiasis
- PMID: 27561392
- PMCID: PMC5000474
- DOI: 10.1038/srep32083
Discovery of a Carbazole-Derived Lead Drug for Human African Trypanosomiasis
Erratum in
-
Erratum: Discovery of a Carbazole-Derived Lead Drug for Human African Trypanosomiasis.Sci Rep. 2017 Jan 11;7:40565. doi: 10.1038/srep40565. Sci Rep. 2017. PMID: 28074881 Free PMC article. No abstract available.
Abstract
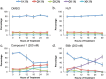
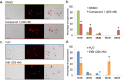
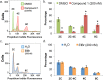
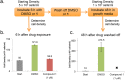
The protozoan parasite Trypanosoma brucei causes the fatal illness human African trypanosomiasis (HAT). Standard of care medications currently used to treat HAT have severe limitations, and there is a need to find new chemical entities that are active against infections of T. brucei. Following a "drug repurposing" approach, we tested anti-trypanosomal effects of carbazole-derived compounds called "Curaxins". In vitro screening of 26 compounds revealed 22 with nanomolar potency against axenically cultured bloodstream trypanosomes. In a murine model of HAT, oral administration of compound 1 cured the disease. These studies established 1 as a lead for development of drugs against HAT. Pharmacological time-course studies revealed the primary effect of 1 to be concurrent inhibition of mitosis coupled with aberrant licensing of S-phase entry. Consequently, polyploid trypanosomes containing 8C equivalent of DNA per nucleus and three or four kinetoplasts were produced. These effects of 1 on the trypanosome are reminiscent of "mitotic slippage" or endoreplication observed in some other eukaryotes.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

