Aurintricarboxylic acid structure modifications lead to reduction of inhibitory properties against virulence factor YopH and higher cytotoxicity
- PMID: 27562597
- PMCID: PMC4999467
- DOI: 10.1007/s11274-016-2123-3
Aurintricarboxylic acid structure modifications lead to reduction of inhibitory properties against virulence factor YopH and higher cytotoxicity
Abstract
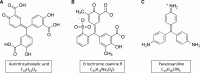
Yersinia sp. bacteria owe their viability and pathogenic virulence to the YopH factor, which is a highly active bacterial protein tyrosine phosphatase. Inhibition of YopH phosphatase results in the lack of Yersinia sp. pathogenicity. We have previously described that aurintricarboxylic acid inhibits the activity of YopH at nanomolar concentrations and represents a unique mechanism of YopH inactivation due to a redox process. This work is a continuation of our previous studies. Here we show that modifications of the structure of aurintricarboxylic acid reduce the ability to inactivate YopH and lead to higher cytotoxicity. In the present paper we examine the inhibitory properties of aurintricarboxylic acid analogues, such as eriochrome cyanine R (ECR) and pararosaniline. Computational docking studies we report here indicate that ATA analogues are not precluded to bind in the YopH active site and in all obtained binding conformations ECR and pararosaniline bind to YopH active site. The free binding energy calculations show that ECR has a stronger binding affinity to YopH than pararosaniline, which was confirmed by experimental YopH enzymatic activity studies. We found that ATA analogues can reversibly reduce the enzymatic activity of YopH, but possess weaker inhibitory properties than ATA. The ATA analogues induced inactivation of YopH is probably due to oxidative mechanism, as pretreatment with catalase prevents from inhibition. We also found that ATA analogues significantly decrease the viability of macrophage cells, especially pararosaniline, while ATA reveals only slight effect on cell viability.
Keywords: Aurintricarboxylic acid (ATA); Eriochrome cyanine R; Pararosaniline; Protein tyrosine phosphatases (PTPs); YopH.
Conflict of interest statement
The authors declare that they have no conflict of interest. Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Figures




References
-
- Achtman M, Morelli G, Zhu P, Wirth T, Diehl I, Kusecek B, Vogler AJ, Wagner DM, Allender CJ, Easterday WR, Chenal-Francisque V, Worsham P, Thomson NR, Parkhill J, Lindler LE, Carniel E, Keim P. Microevolution and history of the plague bacillus, Yersinia pestis. Proc Natl Acad Sci USA. 2004;101:17837–17842. doi: 10.1073/pnas.0408026101. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

