Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents, and young adults (BREATHER): a randomised, open-label, non-inferiority, phase 2/3 trial
- PMID: 27562743
- PMCID: PMC4995440
- DOI: 10.1016/S2352-3018(16)30054-6
Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents, and young adults (BREATHER): a randomised, open-label, non-inferiority, phase 2/3 trial
Abstract
Background: For HIV-1-infected young people facing lifelong antiretroviral therapy (ART), short cycle therapy with long-acting drugs offers potential for drug-free weekends, less toxicity, and better quality-of-life. We aimed to compare short cycle therapy (5 days on, 2 days off ART) versus continuous therapy (continuous ART).
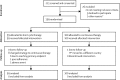
Methods: In this open-label, non-inferiority trial (BREATHER), eligible participants were aged 8-24 years, were stable on first-line efavirenz with two nucleoside reverse transcriptase inhibitors, and had HIV-1 RNA viral load less than 50 copies per mL for 12 months or longer. Patients were randomly assigned (1:1) to remain on continuous therapy or change to short cycle therapy according to a computer-generated randomisation list, with permuted blocks of varying size, stratified by age and African versus non-African sites; the list was prepared by the trial statistician and randomisation was done via a web service accessed by site clinician or one of the three coordinating trials units. The primary outcome was the proportion of participants with confirmed viral load 50 copies per mL or higher at any time up to the 48 week assessment, estimated with the Kaplan-Meier method. The trial was powered to exclude a non-inferiority margin of 12%. Analyses were intention to treat. The trial was registered with EudraCT, number 2009-012947-40, ISRCTN, number 97755073, and CTA, number 27505/0005/001-0001.
Findings: Between April 1, 2011, and June 28, 2013, 199 participants from 11 countries worldwide were randomly assigned, 99 to the short cycle therapy and 100 to continuous therapy, and were followed up until the last patient reached 48 weeks. 105 (53%) were men, median age was 14 years (IQR 12-18), and median CD4 cell count was 735 cells per μL (IQR 576-968). Six (6%) patients assigned to the short cycle therapy versus seven (7%) assigned to continuous therapy had confirmed viral load 50 copies per mL or higher (difference -1·2%, 90% CI -7·3 to 4·9, non-inferiority shown). 13 grade 3 or 4 events occurred in the short cycle therapy group and 14 in the continuous therapy group (p=0·89). Two ART-related adverse events (one gynaecomastia and one spontaneous abortion) occurred in the short cycle therapy group compared with 14 (p=0·02) in the continuous therapy group (five lipodystrophy, two gynaecomastia, one suicidal ideation, one dizziness, one headache and syncope, one spontaneous abortion, one neutropenia, and two raised transaminases).
Interpretation: Non-inferiority of maintaining virological suppression in children, adolescents, and young adults was shown for short cycle therapy versus continous therapy at 48 weeks, with similar resistance and a better safety profile. This short cycle therapy strategy is a viable option for adherent HIV-infected young people who are stable on efavirenz-based ART.
Funding: UK National Institute for Health Research Health Technology Assessment; UK Medical Research Council; European Commission; PENTA Foundation; INSERM SC10-US19, France.
Copyright © 2016 The BREATHER (PENTA 16) Trial Group. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
A breather from daily antiretroviral therapy for adolescents.Lancet HIV. 2016 Sep;3(9):e399-e400. doi: 10.1016/S2352-3018(16)30056-X. Epub 2016 Jun 20. Lancet HIV. 2016. PMID: 27562734 No abstract available.
Similar articles
-
BREATHER (PENTA 16) short-cycle therapy (SCT) (5 days on/2 days off) in young people with chronic human immunodeficiency virus infection: an open, randomised, parallel-group Phase II/III trial.Health Technol Assess. 2016 Jun;20(49):1-108. doi: 10.3310/hta20490. Health Technol Assess. 2016. PMID: 27377073 Free PMC article. Clinical Trial.
-
Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents and young adults (BREATHER): Extended follow-up results of a randomised, open-label, non-inferiority trial.PLoS One. 2018 Apr 23;13(4):e0196239. doi: 10.1371/journal.pone.0196239. eCollection 2018. PLoS One. 2018. PMID: 29684092 Free PMC article. Clinical Trial.
-
Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study.Lancet Infect Dis. 2015 Jul;15(7):793-802. doi: 10.1016/S1473-3099(15)70060-5. Epub 2015 Apr 12. Lancet Infect Dis. 2015. PMID: 25877963 Clinical Trial.
-
Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis.BMC Infect Dis. 2019 May 30;19(1):484. doi: 10.1186/s12879-019-3975-6. BMC Infect Dis. 2019. PMID: 31146698 Free PMC article.
-
[Data on rilpivirine in treatment-naïve patients. Lessons from ECHO, THRIVE and STaR].Enferm Infecc Microbiol Clin. 2013 Jun;31 Suppl 2:20-9. doi: 10.1016/S0213-005X(13)70139-3. Enferm Infecc Microbiol Clin. 2013. PMID: 24252530 Review. Spanish.
Cited by
-
Low unspliced cell-associated HIV RNA in early treated adolescents living with HIV on long suppressive ART.Front Immunol. 2024 Feb 20;15:1334236. doi: 10.3389/fimmu.2024.1334236. eCollection 2024. Front Immunol. 2024. PMID: 38444847 Free PMC article.
-
Pharmacokinetic Model of Tenofovir and Emtricitabine and Their Intracellular Metabolites in Patients in the ANRS 134-COPHAR 3 Trial Using Dose Records.Antimicrob Agents Chemother. 2023 May 17;67(5):e0233918. doi: 10.1128/aac.02339-18. Epub 2023 Apr 26. Antimicrob Agents Chemother. 2023. PMID: 37098914 Free PMC article.
-
Could reduced dosing maintain more people on antiretrovirals after the sudden cuts in USAID funding? A crisis response.AIDS. 2025 Jun 1;39(7):F1-F4. doi: 10.1097/QAD.0000000000004212. Epub 2025 Apr 23. AIDS. 2025. PMID: 40265619 Free PMC article. Review.
-
Efficacy and safety of lower dose tenofovir disoproxil fumarate and efavirenz versus standard dose in HIV-infected, antiretroviral-naive adults: a multicentre, randomized, noninferiority trial.Emerg Microbes Infect. 2020 Dec;9(1):843-850. doi: 10.1080/22221751.2020.1752609. Emerg Microbes Infect. 2020. PMID: 32267205 Free PMC article. Clinical Trial.
-
STACKing the odds for adolescent survival: health service factors associated with full retention in care and adherence amongst adolescents living with HIV in South Africa.J Int AIDS Soc. 2018 Sep;21(9):e25176. doi: 10.1002/jia2.25176. J Int AIDS Soc. 2018. PMID: 30240121 Free PMC article.
References
-
- WHO . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd edn. World Health Organisation; Geneva: 2016. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf (accessed June 10, 2016). - PubMed
-
- British HIV Association British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy. 2015. http://www.bhiva.org/documents/Guidelines/Treatment/2015/2015-treatment-... (accessed Jan 13, 2016). - PubMed
-
- Kim SH, McDonald S, Kim S, Foster C, Fidler S. Importance of self-motivation and social support in medication adherence in HIV-infected adolescents in the United Kingdom and Ireland: a multicentre HYPNet study. AIDS Patient Care STDS. 2015;29:354–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

