Design, Synthesis, and Characterization of Sulfamide and Sulfamate Nucleotidomimetic Inhibitors of hHint1
- PMID: 27563403
- PMCID: PMC4983727
- DOI: 10.1021/acsmedchemlett.6b00169
Design, Synthesis, and Characterization of Sulfamide and Sulfamate Nucleotidomimetic Inhibitors of hHint1
Abstract
Hint1 has recently emerged to be an important target of interest due to its involvement in the regulation of a broad range of CNS functions including opioid signaling, tolerance, neuropathic pain, and nicotine dependence. A series of inhibitors were rationally designed, synthesized, and tested for their inhibitory activity against hHint1 using isothermal titration calorimetry (ITC). The studies resulted in the development of the first small-molecule inhibitors of hHint1 with submicromolar binding affinities. A combination of thermodynamic and high-resolution X-ray crystallographic studies provides an insight into the biomolecular recognition of ligands by hHint1. These novel inhibitors have potential utility as molecular probes to better understand the role and function of hHint1 in the CNS.
Keywords: CNS; ITC; central nervous system; hHint1; human histidine triad nucleotide binding protein 1; isothermal titration calorimetry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
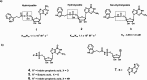




Similar articles
-
Switch-on fluorescent/FRET probes to study human histidine triad nucleotide binding protein 1 (hHint1), a novel target for opioid tolerance and neuropathic pain.Org Biomol Chem. 2017 Dec 13;15(48):10230-10237. doi: 10.1039/c7ob02472j. Org Biomol Chem. 2017. PMID: 29177353
-
Inhibition by divalent metal ions of human histidine triad nucleotide binding protein1 (hHint1), a regulator of opioid analgesia and neuropathic pain.Biochem Biophys Res Commun. 2017 Sep 23;491(3):760-766. doi: 10.1016/j.bbrc.2017.07.111. Epub 2017 Jul 21. Biochem Biophys Res Commun. 2017. PMID: 28739258
-
Histidine triad nucleotide-binding proteins HINT1 and HINT2 share similar substrate specificities and little affinity for the signaling dinucleotide Ap4A.FEBS Lett. 2020 May;594(10):1497-1505. doi: 10.1002/1873-3468.13745. Epub 2020 Feb 17. FEBS Lett. 2020. PMID: 31990367
-
Thermodynamics of protein-ligand interactions: history, presence, and future aspects.J Recept Signal Transduct Res. 2004 Feb;24(1-2):1-52. doi: 10.1081/rrs-120037896. J Recept Signal Transduct Res. 2004. PMID: 15344878 Review.
-
Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition.J Mol Recognit. 1999 Jan-Feb;12(1):3-18. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<3::AID-JMR441>3.0.CO;2-6. J Mol Recognit. 1999. PMID: 10398392 Review.
Cited by
-
Discovery of highly potent SARS-CoV-2 nsp14 methyltransferase inhibitors based on adenosine 5'-carboxamides.RSC Med Chem. 2024 Aug 1;15(10):3469-76. doi: 10.1039/d4md00422a. Online ahead of print. RSC Med Chem. 2024. PMID: 39220762 Free PMC article.
-
Synthesis of 7-benzylguanosine cap-analogue conjugates for eIF4E targeted degradation.Eur J Med Chem. 2019 Mar 15;166:339-350. doi: 10.1016/j.ejmech.2019.01.080. Epub 2019 Jan 31. Eur J Med Chem. 2019. PMID: 30735900 Free PMC article.
-
Tunable Supramolecular Assemblies from Amphiphilic Nucleoside Phosphoramidate Nanofibers by Enzyme Activation.Biomacromolecules. 2018 Jul 9;19(7):2650-2656. doi: 10.1021/acs.biomac.8b00254. Epub 2018 May 1. Biomacromolecules. 2018. PMID: 29689161 Free PMC article.
-
The Many Faces of Histidine Triad Nucleotide Binding Protein 1 (HINT1).ACS Pharmacol Transl Sci. 2023 Jul 20;6(10):1310-1322. doi: 10.1021/acsptsci.3c00079. eCollection 2023 Oct 13. ACS Pharmacol Transl Sci. 2023. PMID: 37854629 Free PMC article. Review.
-
Dynamic Long-Range Interactions Influence Substrate Binding and Catalysis by Human Histidine Triad Nucleotide-Binding Proteins (HINTs), Key Regulators of Multiple Cellular Processes and Activators of Antiviral ProTides.Biochemistry. 2022 Dec 6;61(23):2648-2661. doi: 10.1021/acs.biochem.2c00506. Epub 2022 Nov 18. Biochemistry. 2022. PMID: 36398895 Free PMC article.
References
-
- Garzón J.; Herrero-Labrador R.; Rodríguez-Muñoz M.; Shah R.; Vicente-Sánchez A.; Wagner C. R.; Sánchez-Blázquez P. HINT1 protein: a new therapeutic target to enhance opioid antinociception and block mechanical allodynia. Neuropharmacology 2015, 89, 412–423. 10.1016/j.neuropharm.2014.10.022. - DOI - PubMed
-
- Chou T. F.; Baraniak J.; Kaczmarek R.; Zhou X.; Cheng J.; Ghosh B.; Wagner C. R. Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and Escherichia coli histidine triad nucleotide binding proteins. Mol. Pharmaceutics 2007, 4, 208–217. 10.1021/mp060070y. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

