Use of positron emission tomography scan response to guide treatment change for locally advanced gastric cancer: the Memorial Sloan Kettering Cancer Center experience
- PMID: 27563439
- PMCID: PMC4963363
- DOI: 10.21037/jgo.2016.06.01
Use of positron emission tomography scan response to guide treatment change for locally advanced gastric cancer: the Memorial Sloan Kettering Cancer Center experience
Abstract
Background: Early metabolic response on 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) during neoadjuvant chemotherapy is PET non-responders have poor outcomes whether continuing chemotherapy or proceeding directly to surgery. Use of PET may identify early treatment failure, sparing patients from inactive therapy and allowing for crossover to alternative therapies. We examined the effectiveness of PET directed switching to salvage chemotherapy in the PET non-responders.
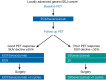
Methods: Patients with locally advanced resectable FDG-avid gastric or gastroesophageal junction (GEJ) adenocarcinoma received bevacizumab 15 mg/kg, epirubicin 50 mg/m(2), cisplatin 60 mg/m(2) day 1, and capecitabine 625 mg/m(2) bid (ECX) every 21 days. PET scan was obtained at baseline and after cycle 1. PET responders, (i.e., ≥35% reduction in FDG uptake at the primary tumor) continued ECX + bev. Non-responders switched to docetaxel 30 mg/m(2), irinotecan 50 mg/mg(2) day 1 and 8 plus bevacizumab every 21 days for 2 cycles. Patients then underwent surgery. The primary objective was to improve the 2-year disease free survival (DFS) from 30% (historical control) to 53% in the non-responders.
Results: Twenty evaluable patients enrolled before the study closed for poor accrual. Eleven were PET responders and the 9 non-responders switched to the salvage regimen. With a median follow-up of 38.2 months, the 2-year DFS was 55% [95% confidence interval (CI), 30-85%] in responders compared with 56% in the non-responder group (95% CI, 20-80%, P=0.93).
Conclusions: The results suggest that changing chemotherapy regimens in PET non-responding patients may improve outcomes. Results from this pilot trial are hypothesis generating and suggest that PET directed neoadjuvant therapy merits evaluation in a larger trial.
Keywords: 18-fluorodeoxyglucose-positron emission tomography (FDG-PET); Gastroesophageal cancer; locally advanced; neoadjuvant chemotherapy.
Conflict of interest statement
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources