Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review
- PMID: 27563457
- PMCID: PMC4963375
- DOI: 10.21037/jgo.2016.05.06
Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review
Abstract
Background: Proton beam radiotherapy (PBT) is frequently shown to be dosimetrically superior to photon radiotherapy (RT), though supporting data for clinical benefit are severely limited. Because of the potential for toxicity reduction in gastrointestinal (GI) malignancies, we systematically reviewed the literature on clinical outcomes (survival/toxicity) of PBT.
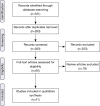
Methods: A systematic search of PubMed, EMBASE, abstracts from meetings of the American Society for Radiation Oncology, Particle Therapy Co-Operative Group, and American Society of Clinical Oncology was conducted for publications from 2000-2015. Thirty-eight original investigations were analyzed.
Results: Although results of PBT are not directly comparable to historical data, outcomes roughly mirror previous data, generally with reduced toxicities for PBT in some neoplasms. For esophageal cancer, PBT is associated with reduced toxicities, postoperative complications, and hospital stay as compared to photon radiation, while achieving comparable local control (LC) and overall survival (OS). In pancreatic cancer, numerical survival for resected/unresected cases is also similar to existing photon data, whereas grade ≥3 nausea/emesis and post-operative complications are numerically lower than those reported with photon RT. The strongest data in support of PBT for HCC comes from phase II trials demonstrating very low toxicities, and a phase III trial of PBT versus transarterial chemoembolization demonstrating trends towards improved LC and progression-free survival (PFS) with PBT, along with fewer post-treatment hospitalizations. Survival and toxicity data for cholangiocarcinoma, liver metastases, and retroperitoneal sarcoma are also roughly equivalent to historical photon controls. There are two small reports for gastric cancer and three for anorectal cancer; these are not addressed further.
Conclusions: Limited quality (and quantity) of data hamper direct comparisons and conclusions. However, the available data, despite the inherent caveats and limitations, suggest that PBT offers the potential to achieve significant reduction in treatment-related toxicities without compromising survival or LC for multiple GI malignancies. Several randomized comparative trials are underway that will provide more definitive answers.
Keywords: Proton radiation therapy (PBT); anal cancer; cholangiocarcinoma; esophageal cancer; gastric cancer; liver cancer; metastases; pancreatic cancer; particle therapy; rectal cancer; retroperitoneal sarcoma.
Conflict of interest statement
Figures
Similar articles
-
Clinical Outcomes and Toxicity of Proton Radiotherapy for Breast Cancer.Clin Breast Cancer. 2016 Jun;16(3):145-54. doi: 10.1016/j.clbc.2016.02.006. Epub 2016 Feb 12. Clin Breast Cancer. 2016. PMID: 26995159 Review.
-
Clinical Outcomes of Proton Radiotherapy for Uveal Melanoma.Clin Oncol (R Coll Radiol). 2016 Aug;28(8):e17-27. doi: 10.1016/j.clon.2016.01.034. Epub 2016 Feb 23. Clin Oncol (R Coll Radiol). 2016. PMID: 26915706
-
Proton radiotherapy for gynecologic neoplasms.Acta Oncol. 2016 Nov;55(11):1257-1265. doi: 10.1080/0284186X.2016.1205218. Epub 2016 Aug 8. Acta Oncol. 2016. PMID: 27500710 Review.
-
Hadrontherapy for cancer. An overview of HTA reports and ongoing studies.Recenti Prog Med. 2019 Dec;110(12):566-586. doi: 10.1701/3278.32516. Recenti Prog Med. 2019. PMID: 31909760 Review. English.
-
Reirradiation With Proton Therapy for Recurrent Malignancies of the Esophagus and Gastroesophageal Junction: Results of the Proton Collaborative Group Multi-Institutional Prospective Registry Trial.Adv Radiat Oncol. 2024 Feb 6;9(5):101459. doi: 10.1016/j.adro.2024.101459. eCollection 2024 May. Adv Radiat Oncol. 2024. PMID: 38596455 Free PMC article.
Cited by
-
Impact of Endoscopic Ultrasonography on 18F-FDG-PET/CT Upfront Towards Patient Specific Esophageal Cancer Treatment.Ann Surg Oncol. 2017 Jul;24(7):1828-1834. doi: 10.1245/s10434-017-5835-1. Epub 2017 Mar 16. Ann Surg Oncol. 2017. PMID: 28303427 Free PMC article.
-
Clinical Outcomes of Proton Beam Therapy for Unresectable Locally Advanced Pancreatic Cancer: A Single-Center Retrospective Study.Adv Radiat Oncol. 2024 Aug 2;9(10):101577. doi: 10.1016/j.adro.2024.101577. eCollection 2024 Oct. Adv Radiat Oncol. 2024. PMID: 39309704 Free PMC article.
-
Current status of radiotherapy for patients with thoracic esophageal cancer in Japan, based on the Comprehensive Registry of Esophageal Cancer in Japan from 2009 to 2011 by the Japan Esophageal Society.Esophagus. 2020 Jan;17(1):25-32. doi: 10.1007/s10388-019-00690-z. Epub 2019 Aug 31. Esophagus. 2020. PMID: 31473871
-
Advances in Radiotherapy Management of Esophageal Cancer.J Clin Med. 2016 Oct 21;5(10):91. doi: 10.3390/jcm5100091. J Clin Med. 2016. PMID: 27775643 Free PMC article. Review.
-
PTCOG Gastrointestinal Subcommittee Lower Gastrointestinal Tract Malignancies Consensus Statement.Int J Part Ther. 2024 Apr 26;11:100019. doi: 10.1016/j.ijpt.2024.100019. eCollection 2024 Mar. Int J Part Ther. 2024. PMID: 38757077 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources