Harnessing the immune system to improve cancer therapy
- PMID: 27563648
- PMCID: PMC4971375
- DOI: 10.21037/atm.2016.04.01
Harnessing the immune system to improve cancer therapy
Abstract
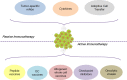
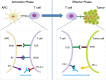
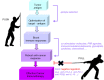
Cancer immunotherapy uses the immune system and its components to mount an anti-tumor response. During the last decade, it has evolved from a promising therapy option to a robust clinical reality. Many immunotherapeutic modalities are already approved by the Food and Drug Administration (FDA) for treating cancer patients and many others are in the pipeline for approval as standalone or combinatorial therapeutic interventions, several also combined with standard treatments in clinical studies. The two main axes of cancer immunotherapeutics refer to passive and active treatments. Prominent examples of passive immunotherapy include administration of monoclonal antibodies and cytokines and adoptive cell transfer of ex vivo "educated" immune cells. Active immunotherapy refers, among others, to anti-cancer vaccines [peptide, dendritic cell (DC)-based and allogeneic whole cell vaccines], immune checkpoint inhibitors and oncolytic viruses, whereas new approaches that can further enhance anti-cancer immune responses are also widely explored. Herein, we present the most popular cancer immunotherapy approaches and discuss their clinical relevance referring to data acquired from clinical trials. To date, clinical experience and efficacy suggest that combining more than one immunotherapy interventions, in conjunction with other treatment options like chemotherapy, radiotherapy and targeted or epigenetic therapy, should guide the way to cancer cure.
Keywords: Cancer immunotherapy; checkpoint inhibitors; dendritic cells (DCs); monoclonal antibodies (mAbs); peptide vaccines.
Conflict of interest statement
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
