Efficient Synthesis of Fully Substituted Pyrrolidine-Fused 3-Spirooxindoles via 1,3-Dipolar Cycloaddition of Aziridine and 3-Ylideneoxindole
- PMID: 27563867
- PMCID: PMC6274301
- DOI: 10.3390/molecules21091113
Efficient Synthesis of Fully Substituted Pyrrolidine-Fused 3-Spirooxindoles via 1,3-Dipolar Cycloaddition of Aziridine and 3-Ylideneoxindole
Abstract
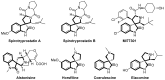
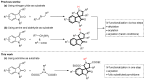
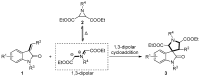
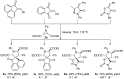
Drug-like spirocyclic scaffolds have been prepared by fusing fully functionalized pyrrolidine with oxindoles in an approach based on 1,3-dipolar cycloaddition. Reaction between aziridine and 3-ylideneoxindole generated diverse spirooxindole-pyrrolidines in good yield (up to 95%) with high diastereoselectivity (up to >20:1). The reaction also proceeded smoothly with several other synthetically useful activated trisubstituted olefins. The mild reaction conditions, short reaction times, and high tolerance for various substitutions make this approach attractive for constructing pharmacologically interesting spiro-architectures.
Keywords: 1,3-dipolar cycloaddition; aziridine; single-step reaction; spirooxindole-pyrrolidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

