Identification of a New Morpholine Scaffold as a P2Y12 Receptor Antagonist
- PMID: 27563870
- PMCID: PMC6273072
- DOI: 10.3390/molecules21091114
Identification of a New Morpholine Scaffold as a P2Y12 Receptor Antagonist
Abstract
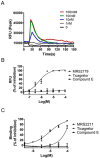
The P2Y12 receptor is critical for platelet activation and is an attractive drug target for the prevention of atherothrombotic events. Despite the proven antithrombotic efficacy of P2Y12 inhibitors, these thienopyridine scaffolds are prodrugs that lack important features of the ideal antithrombotic agent. For this reason, ticagrelor-a new chemical class of P2Y12 receptor antagonist-was developed, but it can cause shortness of breath and various types of bleeding. Moreover, ticagrelor is a cytochrome P450 3A4 substrate/inhibitor and, therefore, caution should be exercised when it is used concomitantly with strong CYP3A4 inducers/inhibitors. There is a need for novel P2Y12 receptor antagonist scaffolds that are reversible and have high efficacy without associated side effects. Here, we describe a novel antagonist containing a morpholine moiety that was identified by screening libraries of commercially available compounds. The molecule, Compound E, acted on P2Y12, but not P2Y1 and P2Y13, and exhibited pharmacological characteristics that were distinct from those of ticagrelor, acting instead on P2Y12 via an allosteric mechanism. These results provide a basis for the development/optimization of a new class of P2Y12 antagonists.
Keywords: P2Y12 receptor antagonist; antiplatelet; morpholine moiety; ticagrelor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

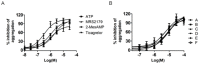

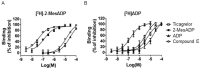
References
-
- Shaver S.R. P2Y receptors: Biological advances and therapeutic opportunities. Curr. Opin. Drug Discov. Dev. 2001;4:665–670. - PubMed
-
- Committee C.S. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

