SIRT1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-E1 cells exposed to sodium fluoride
- PMID: 27564107
- PMCID: PMC5323150
- DOI: 10.18632/oncotarget.11573
SIRT1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-E1 cells exposed to sodium fluoride
Abstract
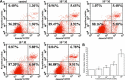
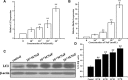
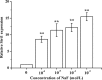
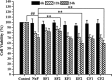
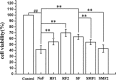
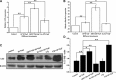
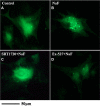
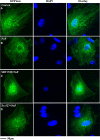
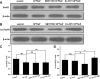
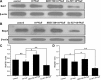
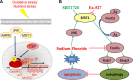
Fluorine may result in damage to teeth, bones and other body tissues, and is a serious public health problem. SIRT1 deacetylates FOXOs, which brings about apoptosis and autophagy promotion or suppression. Fluorine may induce cell apoptosis, however, the role of autophagy in apoptosis induced by fluorine is still poorly understood, and the interaction between SIRT1 and FOXOs should be further illustrated. Therefore, this study investigated the mechanisms underlying the NaF- induced apoptosis and autophagy in osteoblast-like MC3T3-E1 cells in vitro through activating or inhibiting SIRT1. Via RT-PCR, western blot, flow cytometry assays, fluorescence and laser confocal microscopy, it was found that NaF induced both cell apoptosis and autophagy. Results also showed that NaF up-regulated SIRT1 expression in a dose-dependent manner. The autophagy of MC3T3-E1 was also up- regulated indirectly whilst apoptosis was significantly attenuated when incubated with the SIRT1 activator SRT1720. When SIRT1 inhibitor Ex-527 was used, the latter effects were reversed. Furthermore, SIRT1 increased deacetylation of FoxO1 and promoted the up-regulation of its target substrate Rab7, as well as increase of Bnip3 which was substrate of FoxO3, and we hypothesize that these pathways may cause an increase in autophagic flux and a reduction in apoptosis. In conclusion, SIRT1-induced autophagy enhancement protects against fluoride-induced apoptosis through autophagy induction in MC3T3-E1 cells, which may be associated with a SIRT1-FoxO1-Rab7 axis and a SIRT1-FoxO3-Binp3 axis. The role of SIRT1 in selecting between cell survival and death provides a potential therapeutic strategy in fluorosis.
Keywords: MC3T3-E1 cells; SIRT1; apoptosis; autophagy; sodium fluoride.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
SIRT1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-E1 cells exposed to fluoride.Toxicol In Vitro. 2019 Jun;57:28-38. doi: 10.1016/j.tiv.2019.02.006. Epub 2019 Feb 7. Toxicol In Vitro. 2019. PMID: 30738887
-
Sodium fluoride induces apoptosis and autophagy via the endoplasmic reticulum stress pathway in MC3T3-E1 osteoblastic cells.Mol Cell Biochem. 2019 Apr;454(1-2):77-85. doi: 10.1007/s11010-018-3454-1. Epub 2018 Dec 5. Mol Cell Biochem. 2019. PMID: 30519783
-
TGF-β1 acts as mediator in fluoride-induced autophagy in the mouse osteoblast cells.Food Chem Toxicol. 2018 May;115:26-33. doi: 10.1016/j.fct.2018.02.065. Epub 2018 Mar 2. Food Chem Toxicol. 2018. PMID: 29505816
-
SIRT1-FOXOs activity regulates diabetic complications.Pharmacol Res. 2022 Jan;175:106014. doi: 10.1016/j.phrs.2021.106014. Epub 2021 Nov 29. Pharmacol Res. 2022. PMID: 34856334 Review.
-
Targeting SIRT1-regulated autophagic cell death as a novel therapeutic avenue for cancer prevention.Drug Discov Today. 2023 Sep;28(9):103692. doi: 10.1016/j.drudis.2023.103692. Epub 2023 Jun 26. Drug Discov Today. 2023. PMID: 37379905 Review.
Cited by
-
Paeoniflorin Attenuates Dexamethasone-Induced Apoptosis of Osteoblast Cells and Promotes Bone Formation via Regulating AKT/mTOR/Autophagy Signaling Pathway.Evid Based Complement Alternat Med. 2021 Apr 7;2021:6623464. doi: 10.1155/2021/6623464. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33880124 Free PMC article.
-
Phosphorylated GSK‑3β protects stress‑induced apoptosis of myoblasts via the PI3K/Akt signaling pathway.Mol Med Rep. 2020 Jul;22(1):317-327. doi: 10.3892/mmr.2020.11105. Epub 2020 Apr 30. Mol Med Rep. 2020. PMID: 32377749 Free PMC article.
-
Resveratrol alleviate the injury of mice liver induced by cadmium sulfide nanoparticles.Kaohsiung J Med Sci. 2019 May;35(5):297-302. doi: 10.1002/kjm2.12056. Epub 2019 Mar 26. Kaohsiung J Med Sci. 2019. PMID: 30913377 Free PMC article.
-
Regulation of osteoblast behaviors via cross-talk between Hippo/YAP and MAPK signaling pathway under fluoride exposure.J Mol Med (Berl). 2019 Jul;97(7):1003-1017. doi: 10.1007/s00109-019-01785-x. Epub 2019 May 4. J Mol Med (Berl). 2019. PMID: 31055605
-
Sodium hydrosulfide alleviates dexamethasone-induced cell senescence and dysfunction through targeting the miR-22/sirt1 pathway in osteoblastic MC3T3-E1 cells.Exp Ther Med. 2021 Mar;21(3):238. doi: 10.3892/etm.2021.9669. Epub 2021 Jan 21. Exp Ther Med. 2021. PMID: 33603846 Free PMC article.
References
-
- Wu DS, Zheng BS, Tang XY, Li SH, Wang BB, Wang MS. Fluorine in Chinese coals. Fluoride. 2004;37:125–132.
-
- Anuradha CD, Kanno KS, Hirano S. Oxidative damage to mitochondria is a preliminary step to caspase-3 activation in fluoride-induced apoptosis in HL-60 cells. Free Radic Biol Med. 2001;31:367–373. - PubMed
-
- Lee JH, Jung JY, Jeong YJ, Park JH, Yang KH, Choi NK, Kim SH, Kim WJ. Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology. 2008;243:340–347. - PubMed
-
- Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014;19:555–566. - PubMed
-
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

