Paradoxical overexpression of MBNL2 in hepatocellular carcinoma inhibits tumor growth and invasion
- PMID: 27564110
- PMCID: PMC5323177
- DOI: 10.18632/oncotarget.11577
Paradoxical overexpression of MBNL2 in hepatocellular carcinoma inhibits tumor growth and invasion
Abstract
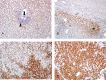
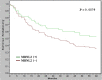
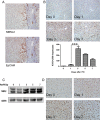
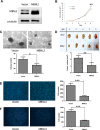
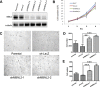
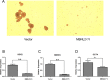
Pre-mRNA alternative splicing is an essential step in the process of gene expression. It provides cells with the opportunity to create various protein isoforms. Disruptions of alternative splicing are associated with various diseases, including cancer. The muscleblind-like (MBNL) protein is a splicing regulatory protein. Overexpression of MBNL proteins in embryonic stem cells promotes differentiated cell-like alternative splicing patterns. We examined the expression level of MBNL2 in 143 resected HCCs using immunohistochemistry. MBNL2 was overexpressed in 51 (35.7%) HCCs. The overexpression of MBNL2 correlated with smaller tumor size (≤ 3 cm, P = 0.0108) and low tumor stage (Stage I, P = 0.0026), indicating that MBNL2 expression was lost in the late stage of HCC development. Furthermore, patients with MBNL2-positive HCCs had a borderline better 5-year overall survival (P = 0.0579). In non-cancerous liver parenchyma, MBNL2 was stained on the Canals of Hering and hepatocytes newly derived from hepatic progenitor cells. The overexpression of MBNL2 in Hep-J5 cells suppressed proliferation, tumorsphere formation, migration, and in vitro invasion, and also reduced in vivo tumor growth in NOD/SCID mice. In contrast, MBNL2 depletion with RNA interference in Huh7 cells increased in vitro migration and invasion, but did not enhance tumor growth. These results indicate that MBNL2 is a tumor suppressor in hepatocarcinogenesis.
Keywords: alternative splicing; hepatic progenitor cells; hepatocellular carcinoma; liver carcinogenesis; muscleblind proteins.
Conflict of interest statement
No potential conflicts of interest was disclosed.
Figures
Similar articles
-
The suppressor of cytokine signaling 2 (SOCS2) inhibits tumor metastasis in hepatocellular carcinoma.Tumour Biol. 2016 Oct;37(10):13521-13531. doi: 10.1007/s13277-016-5215-7. Epub 2016 Jul 28. Tumour Biol. 2016. PMID: 27465557
-
Lin-28B expression promotes transformation and invasion in human hepatocellular carcinoma.Carcinogenesis. 2010 Sep;31(9):1516-22. doi: 10.1093/carcin/bgq107. Epub 2010 Jun 4. Carcinogenesis. 2010. PMID: 20525879
-
MicroRNA-137 represses FBI-1 to inhibit proliferation and in vitro invasion and migration of hepatocellular carcinoma cells.Tumour Biol. 2016 Oct;37(10):13995-14008. doi: 10.1007/s13277-016-5230-8. Epub 2016 Aug 4. Tumour Biol. 2016. PMID: 27492460
-
Fibulin-5 inhibits hepatocellular carcinoma cell migration and invasion by down-regulating matrix metalloproteinase-7 expression.BMC Cancer. 2014 Dec 12;14:938. doi: 10.1186/1471-2407-14-938. BMC Cancer. 2014. PMID: 25494879 Free PMC article.
-
HUS1 checkpoint clamp component (HUS1) is a potential tumor suppressor in primary hepatocellular carcinoma.Mol Carcinog. 2019 Jan;58(1):76-87. doi: 10.1002/mc.22908. Epub 2018 Sep 19. Mol Carcinog. 2019. PMID: 30182378
Cited by
-
ERK Inhibitor Ulixertinib Inhibits High-Risk Neuroblastoma Growth In Vitro and In Vivo.Cancers (Basel). 2022 Nov 10;14(22):5534. doi: 10.3390/cancers14225534. Cancers (Basel). 2022. PMID: 36428626 Free PMC article.
-
Competitive Endogenous RNA Landscape in Epstein-Barr Virus Associated Nasopharyngeal Carcinoma.Front Cell Dev Biol. 2021 Nov 4;9:782473. doi: 10.3389/fcell.2021.782473. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34805186 Free PMC article.
-
Muscleblind-like 2 controls the hypoxia response of cancer cells.RNA. 2020 May;26(5):648-663. doi: 10.1261/rna.073353.119. Epub 2020 Mar 3. RNA. 2020. PMID: 32127384 Free PMC article.
-
Messenger RNA Life-Cycle in Cancer Cells: Emerging Role of Conventional and Non-Conventional RNA-Binding Proteins?Int J Mol Sci. 2018 Feb 25;19(3):650. doi: 10.3390/ijms19030650. Int J Mol Sci. 2018. PMID: 29495341 Free PMC article. Review.
-
RNA-binding Protein MBNL2 regulates Cancer Cell Metastasis through MiR-182-MBNL2-AKT Pathway.J Cancer. 2021 Sep 21;12(22):6715-6726. doi: 10.7150/jca.62816. eCollection 2021. J Cancer. 2021. PMID: 34659561 Free PMC article.
References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Hsu HC, Huang AM, Lai PL, Chien WM, Peng SY, Lin SW. Genetic alterations at the splice junction of p53 gene in human hepatocellular carcinoma. Hepatology. 1994;19:122–128. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

