Identification of miRNAs Potentially Involved in Bronchiolitis Obliterans Syndrome: A Computational Study
- PMID: 27564214
- PMCID: PMC5001701
- DOI: 10.1371/journal.pone.0161771
Identification of miRNAs Potentially Involved in Bronchiolitis Obliterans Syndrome: A Computational Study
Abstract
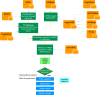
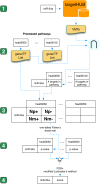
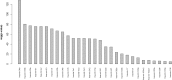
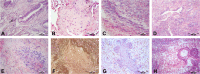
The pathogenesis of Bronchiolitis Obliterans Syndrome (BOS), the main clinical phenotype of chronic lung allograft dysfunction, is poorly understood. Recent studies suggest that epigenetic regulation of microRNAs might play a role in its development. In this paper we present the application of a complex computational pipeline to perform enrichment analysis of miRNAs in pathways applied to the study of BOS. The analysis considered the full set of miRNAs annotated in miRBase (version 21), and applied a sequence of filtering approaches and statistical analyses to reduce this set and to score the candidate miRNAs according to their potential involvement in BOS development. Dysregulation of two of the selected candidate miRNAs-miR-34a and miR-21 -was clearly shown in in-situ hybridization (ISH) on five explanted human BOS lungs and on a rat model of acute and chronic lung rejection, thus definitely identifying miR-34a and miR-21 as pathogenic factors in BOS and confirming the effectiveness of the computational pipeline.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




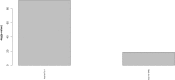

Similar articles
-
miRNAs Potentially Involved in Post Lung Transplant-Obliterative Bronchiolitis: The Role of miR-21-5p.Cells. 2021 Mar 20;10(3):688. doi: 10.3390/cells10030688. Cells. 2021. PMID: 33804639 Free PMC article.
-
Serum miRNAs as potential biomarkers for the bronchiolitis obliterans syndrome after lung transplantation.Transpl Immunol. 2017 Jun;42:1-4. doi: 10.1016/j.trim.2017.04.002. Epub 2017 Apr 28. Transpl Immunol. 2017. PMID: 28457921 Clinical Trial.
-
Dysregulated MicroRNA Expression and Chronic Lung Allograft Rejection in Recipients With Antibodies to Donor HLA.Am J Transplant. 2015 Jul;15(7):1933-47. doi: 10.1111/ajt.13185. Epub 2015 Feb 3. Am J Transplant. 2015. PMID: 25649290 Free PMC article.
-
Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation.Pediatr Transplant. 2005 Feb;9(1):84-93. doi: 10.1111/j.1399-3046.2004.00270.x. Pediatr Transplant. 2005. PMID: 15667618 Review.
-
Precision medicine: integration of genetics and functional genomics in prediction of bronchiolitis obliterans after lung transplantation.Curr Opin Pulm Med. 2019 May;25(3):308-316. doi: 10.1097/MCP.0000000000000579. Curr Opin Pulm Med. 2019. PMID: 30883449 Review.
Cited by
-
Markers of Bronchiolitis Obliterans Syndrome after Lung Transplant: Between Old Knowledge and Future Perspective.Biomedicines. 2022 Dec 17;10(12):3277. doi: 10.3390/biomedicines10123277. Biomedicines. 2022. PMID: 36552035 Free PMC article. Review.
-
Regulation of Endothelial-to-Mesenchymal Transition by MicroRNAs in Chronic Allograft Dysfunction.Transplantation. 2019 Apr;103(4):e64-e73. doi: 10.1097/TP.0000000000002589. Transplantation. 2019. PMID: 30907855 Free PMC article.
-
miRNAs Potentially Involved in Post Lung Transplant-Obliterative Bronchiolitis: The Role of miR-21-5p.Cells. 2021 Mar 20;10(3):688. doi: 10.3390/cells10030688. Cells. 2021. PMID: 33804639 Free PMC article.
References
-
- Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002. August;166(4):440–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

