Triheptanoin Protects Motor Neurons and Delays the Onset of Motor Symptoms in a Mouse Model of Amyotrophic Lateral Sclerosis
- PMID: 27564703
- PMCID: PMC5001695
- DOI: 10.1371/journal.pone.0161816
Triheptanoin Protects Motor Neurons and Delays the Onset of Motor Symptoms in a Mouse Model of Amyotrophic Lateral Sclerosis
Abstract
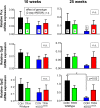
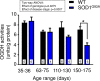
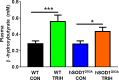
There is increasing evidence that energy metabolism is disturbed in Amyotrophic Lateral Sclerosis (ALS) patients and animal models. Treatment with triheptanoin, the triglyceride of heptanoate, is a promising approach to provide alternative fuel to improve oxidative phosphorylation and aid ATP generation. Heptanoate can be metabolized to propionyl-CoA, which after carboxylation can produce succinyl-CoA and thereby re-fill the tricarboxylic acid (TCA) cycle (anaplerosis). Here we tested the hypothesis that treatment with triheptanoin prevents motor neuron loss and delays the onset of disease symptoms in female mice overexpressing the mutant human SOD1G93A (hSOD1G93A) gene. When oral triheptanoin (35% of caloric content) was initiated at P35, motor neuron loss at 70 days of age was attenuated by 33%. In untreated hSOD1G93A mice, the loss of hind limb grip strength began at 16.7 weeks. Triheptanoin maintained hind limb grip strength for 2.8 weeks longer (p<0.01). Loss of balance on the rotarod and reduction of body weight were delayed by 13 and 11 days respectively (both p<0.01). Improved motor function occurred in parallel with alterations in the expression of genes associated with muscle metabolism. In gastrocnemius muscles, the mRNA levels of pyruvate, 2-oxoglutarate and succinate dehydrogenases and methyl-malonyl mutase were reduced by 24-33% in 10 week old hSOD1G93A mice when compared to wild-type mice, suggesting that TCA cycling in skeletal muscle may be slowed in this ALS mouse model at a stage when muscle strength is still normal. At 25 weeks of age, mRNA levels of succinate dehydrogenases, glutamic pyruvic transaminase 2 and the propionyl carboxylase β subunit were reduced by 69-84% in control, but not in triheptanoin treated hSOD1G93A animals. Taken together, our results suggest that triheptanoin slows motor neuron loss and the onset of motor symptoms in ALS mice by improving TCA cycling.
Conflict of interest statement
We have applied for a US patent regarding the treatment of ALS (Neurodegenerative disorders and methods of treatment and diagnosis thereof. US provisional patent application 2013; US 61/904,365). Sasol and Ultragenyx Pharmaceuticals Inc. donated triheptanoin. There are no additional patents, products in development or marketed products to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures






Similar articles
-
Alternative Fuels in Epilepsy and Amyotrophic Lateral Sclerosis.Neurochem Res. 2017 Jun;42(6):1610-1620. doi: 10.1007/s11064-016-2106-7. Epub 2016 Nov 21. Neurochem Res. 2017. PMID: 27868154 Review.
-
Triheptanoin partially restores levels of tricarboxylic acid cycle intermediates in the mouse pilocarpine model of epilepsy.J Neurochem. 2014 Apr;129(1):107-19. doi: 10.1111/jnc.12610. Epub 2013 Dec 2. J Neurochem. 2014. PMID: 24236946
-
Triheptanoin protects against status epilepticus-induced hippocampal mitochondrial dysfunctions, oxidative stress and neuronal degeneration.J Neurochem. 2018 Feb;144(4):431-442. doi: 10.1111/jnc.14275. Epub 2018 Jan 3. J Neurochem. 2018. PMID: 29222946
-
Motor neuron dysfunction in a mouse model of ALS: gender-dependent effect of P2X7 antagonism.Toxicology. 2013 Sep 6;311(1-2):69-77. doi: 10.1016/j.tox.2013.04.004. Epub 2013 Apr 11. Toxicology. 2013. PMID: 23583883
-
Triheptanoin--a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment?Epilepsy Res. 2012 Jul;100(3):239-44. doi: 10.1016/j.eplepsyres.2011.05.023. Epub 2011 Aug 19. Epilepsy Res. 2012. PMID: 21855298 Free PMC article. Review.
Cited by
-
Swim Training Affects on Muscle Lactate Metabolism, Nicotinamide Adenine Dinucleotides Concentration, and the Activity of NADH Shuttle Enzymes in a Mouse Model of Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2022 Sep 29;23(19):11504. doi: 10.3390/ijms231911504. Int J Mol Sci. 2022. PMID: 36232801 Free PMC article.
-
Enhanced cerebral branched-chain amino acid metabolism in R6/2 mouse model of Huntington's disease.Cell Mol Life Sci. 2019 Jun;76(12):2449-2461. doi: 10.1007/s00018-019-03051-2. Epub 2019 Mar 4. Cell Mol Life Sci. 2019. PMID: 30830240 Free PMC article.
-
Current potential therapeutics of amyotrophic lateral sclerosis.Front Neurol. 2024 Apr 24;15:1402962. doi: 10.3389/fneur.2024.1402962. eCollection 2024. Front Neurol. 2024. PMID: 38721118 Free PMC article. Review.
-
Alternative Fuels in Epilepsy and Amyotrophic Lateral Sclerosis.Neurochem Res. 2017 Jun;42(6):1610-1620. doi: 10.1007/s11064-016-2106-7. Epub 2016 Nov 21. Neurochem Res. 2017. PMID: 27868154 Review.
-
Metabolic Dysregulation in Amyotrophic Lateral Sclerosis: Challenges and Opportunities.Curr Genet Med Rep. 2017 Jun;5(2):108-114. doi: 10.1007/s40142-017-0123-8. Epub 2017 Apr 28. Curr Genet Med Rep. 2017. PMID: 29057168 Free PMC article.
References
-
- Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nature reviews Neuroscience 2: 806–819. - PubMed
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

