Adaptive immunity programmes in breast cancer
- PMID: 27564847
- PMCID: PMC5341497
- DOI: 10.1111/imm.12664
Adaptive immunity programmes in breast cancer
Abstract
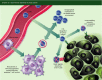
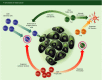
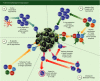
The role of the immune system in shaping cancer development and patient prognosis has recently become an area of intense focus in industry and academia. Harnessing the adaptive arm of the immune system for tumour eradication has shown great promise in a variety of tumour types. Differences between tissues, however, necessitate a greater understanding of the adaptive immunity programmes that are active within each tumour type. In breast cancer, adaptive immune programmes play diverse roles depending on the cellular infiltration found in each tumour. Cytotoxic T lymphocytes and T helper type 1 cells can induce tumour eradication, whereas regulatory T cells and T helper type 2 cells are known to be involved in tumour-promoting immunosuppressive responses. Complicating these matters, heterogeneous expression of hormone receptors and growth factors in different tumours leads to disparate, patient-specific adaptive immune responses. Despite this non-conformity in adaptive immune behaviours, encouraging basic and clinical results have been observed that suggest a role for immunotherapeutic approaches in breast cancer. Here, we review the literature pertaining to the adaptive immune response in breast cancer, summarize the primary findings relating to the breast tumour's biology, and discuss potential clinical immunotherapies.
Keywords: T cells; adaptive immunity; breast cancer; immunotherapy; neoantigens.
© 2016 John Wiley & Sons Ltd.
Figures
References
-
- American Cancer Society . Facts & Figures 2016. Atlanta, GA: American Cancer Society, 2016.
-
- National Cancer Institute; Surveillance, Epidemiology, and End Results (SEER) Program . Stat Fact Sheets: Female Breast Cancer. Bethesda, MD: National Cancer Institute; Surveillance, Epidemiology, and End Results Program, 2016.
-
- Cimino‐Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology 2015; 29:375–85. - PubMed
-
- Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 2000; 343:338–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

