TFEB-amplified Renal Cell Carcinomas: An Aggressive Molecular Subset Demonstrating Variable Melanocytic Marker Expression and Morphologic Heterogeneity
- PMID: 27565001
- PMCID: PMC5069163
- DOI: 10.1097/PAS.0000000000000720
TFEB-amplified Renal Cell Carcinomas: An Aggressive Molecular Subset Demonstrating Variable Melanocytic Marker Expression and Morphologic Heterogeneity
Abstract
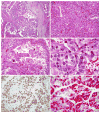
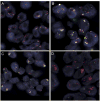
Renal cell carcinomas (RCCs) with the t(6;11)(p21;q12) chromosome translocation are low-grade RCC which often occur in young patients. They typically feature an unusual biphasic morphology characterized by nests of larger epithelioid cells surrounding intraluminal collections of smaller cells clustered around basement membrane material. The t(6;11)(p21;q12) translocation fuses the Alpha (MALAT1) gene with the TFEB transcription factor gene, resulting in upregulated expression of intact native TFEB that drives the aberrant expression of melanocytic markers which is a hallmark of this distinctive neoplasm. We now report 8 cases of RCC, which demonstrate TFEB gene amplification (6 without TFEB rearrangement, 2 with concurrent TFEB rearrangement) and demonstrate downstream consequences of TFEB overexpression. Like the unamplified t(6;11) RCC, all TFEB-amplified RCC were associated with aberrant melanocytic marker expression. However, several differences between TFEB-amplified RCC and the usual unamplified t(6;11) RCC are evident. First, TFEB-amplified RCC occurred in older patients (median age, 64.5 y) compared with unamplified t(6;11) RCC (median age, 31 y). Second, the morphology of TFEB-amplified RCC is not entirely distinctive, frequently featuring nests of high-grade epithelioid cells with eosinophilic cytoplasm associated with pseudopapillary formation and necrosis, or true papillary formations. These patterns raise the differential diagnosis of high-grade clear cell and papillary RCC. Third, TFEB and melanocytic marker expression was more variable within the TFEB-amplified RCC. TFEB protein expression by immunohistochemistry was detectable in 6 of 8 cases. While all 8 cases expressed melan-A, only 5 of 8 expressed cathepsin K and only 3 of 8 expressed HMB45. Fourth, the TFEB-amplified RCC were associated with a more aggressive clinical course; 3 of 8 cases presented with advanced stage or metastatic disease, 2 subsequently developed metastatic disease, whereas the other 3 cases had minimal/no follow-up. Our results are corroborated by scant data reported on 6 TFEB-amplified RCC in the literature, gleaned from 1 case report, 1 abstract, and 4 individual cases identified within 2 genomic studies of large cohorts of RCC. In summary, TFEB-amplified RCC represent a distinct molecular subtype of high-grade adult RCC associated with aggressive clinical behavior, variable morphology, and aberrant melanocytic marker expression.
Figures




References
-
- Argani P, Lae M, Hutchinson B, et al. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific Alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005;29:230–240. - PubMed
-
- Smith N, Illei P, Allaf M, Gonzalez N, Morris K, Hicks J, De Marzo A, Reuter VE, Amin MB, Epstein JI, Netto G, Argani P. t(6;11) renal cell carcinoma (RCC): Expanded immunohistochemical profile exphasizing novel RCC markers and report of ten new genetically-confirmed cases. Am J Surg Pathol. 2014;38:604–14. - PMC - PubMed
-
- Rao Q, Liu B, Cheng L, et al. Renal cell carcinomas with t(6;11)(p21;q12): A clinicopathologic study emphasizing unusual morphology, novel Alpha-TFEB gene fusion point, immunobiomarkers, and ultrastructural features, as well as detection of the gene fusion by fluorescence in situ hybridization. Am J Surg Pathol. 2012;36:1327–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

