Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts
- PMID: 27565350
- PMCID: PMC5167540
- DOI: 10.1016/j.cell.2016.07.041
Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts
Abstract
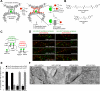
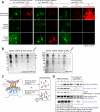
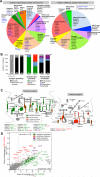
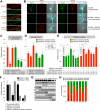
Cellular compartments that cannot be biochemically isolated are challenging to characterize. Here we demonstrate the proteomic characterization of the synaptic clefts that exist at both excitatory and inhibitory synapses. Normal brain function relies on the careful balance of these opposing neural connections, and understanding how this balance is achieved relies on knowledge of their protein compositions. Using a spatially restricted enzymatic tagging strategy, we mapped the proteomes of two of the most common excitatory and inhibitory synaptic clefts in living neurons. These proteomes reveal dozens of synaptic candidates and assign numerous known synaptic proteins to a specific cleft type. The molecular differentiation of each cleft allowed us to identify Mdga2 as a potential specificity factor influencing Neuroligin-2's recruitment of presynaptic neurotransmitters at inhibitory synapses.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Boyken J, Grønborg M, Riedel D, Urlaub H, Jahn R, Chua JJE. Molecular profiling of synaptic vesicle docking sites reveals novel proteins but few differences between glutamatergic and GABAergic synapses. Neuron. 2013;78:285–297. - PubMed
-
- Chapman-Smith A, Cronan JE. In vivo enzymatic protein biotinylation. Biomol. Eng. 1999;16:119–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

