Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism
- PMID: 27565352
- PMCID: PMC5030821
- DOI: 10.1016/j.cell.2016.07.040
Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism
Abstract
Mitochondria house metabolic pathways that impact most aspects of cellular physiology. While metabolite profiling by mass spectrometry is widely applied at the whole-cell level, it is not routinely possible to measure the concentrations of small molecules in mammalian organelles. We describe a method for the rapid and specific isolation of mitochondria and use it in tandem with a database of predicted mitochondrial metabolites ("MITObolome") to measure the matrix concentrations of more than 100 metabolites across various states of respiratory chain (RC) function. Disruption of the RC reveals extensive compartmentalization of mitochondrial metabolism and signatures unique to the inhibition of each RC complex. Pyruvate enables the proliferation of RC-deficient cells but has surprisingly limited effects on matrix contents. Interestingly, despite failing to restore matrix NADH/NAD balance, pyruvate does increase aspartate, likely through the exchange of matrix glutamate for cytosolic aspartate. We demonstrate the value of mitochondrial metabolite profiling and describe a strategy applicable to other organelles.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
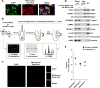
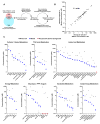
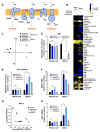
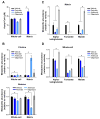
Comment in
-
The Whole (Cell) Is Less Than the Sum of Its Parts.Cell. 2016 Aug 25;166(5):1078-1079. doi: 10.1016/j.cell.2016.08.011. Cell. 2016. PMID: 27565337 Free PMC article.
References
-
- Berry MN, Barritt GJ, Edwards AM, Burdon RH. Isolated Hepatocytes: Preparation, Properties and Applications: Preparation, Properties and Applications. Elsevier Science; 1991.
-
- Bestwick RK, Moffett GL, Mathews CK. Selective expansion of mitochondrial nucleoside triphosphate pools in antimetabolite-treated HeLa cells. Journal of Biological Chemistry. 1982;257:9300–9304. - PubMed
-
- Bogenhagen D, Clayton DA. The Number of Mitochondrial Deoxyribonucleic Acid Genomes in Mouse L and Human HeLa Cells: QUANTITATIVE ISOLATION OF MITOCHONDRIAL DEOXYRIBONUCLEIC ACID. Journal of Biological Chemistry. 1974;249:7991–7995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

