Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: A phase III randomized controlled study
- PMID: 27565435
- PMCID: PMC5215516
- DOI: 10.1080/21645515.2016.1212143
Safety and immunogenicity of an intramuscular quadrivalent influenza vaccine in children 3 to 8 y of age: A phase III randomized controlled study
Abstract
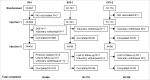
A quadrivalent, inactivated, split-virion influenza vaccine containing a strain from both B lineages (IIV4) has been developed, but its safety and immunogenicity in young children has not been described. This was a phase III, randomized, double-blind, active-controlled, multi-center study to examine the immunogenicity and safety of IIV4 in children 3-8 y of age (EudraCT no. 2011-005374-33). Participants were randomized 5:1:1 to receive the 2013/2014 Northern Hemisphere formulation of IIV4, an investigational trivalent comparator (IIV3) containing the B/Victoria lineage strain, or the licensed Northern Hemisphere IIV3 containing the B/Yamagata lineage strain. Participants who had not previously received a full influenza vaccination schedule received 2 doses of vaccine 28 d apart; all others received a single dose. 1242 children were included. For all 4 strains, IIV4 induced geometric mean haemagglutination inhibition titres non-inferior to those induced by the IIV3 comparators. For both B strains, geometric mean antibody titres induced by IIV4 were superior to those induced by the IIV3 with the alternative lineage strain. Similar proportions of participants vaccinated with IIV4 and IIV3 reported solicited injection-site reactions, solicited systemic reactions, and vaccine-related adverse events. A single vaccine-related serious adverse event, thrombocytopenia, was reported 9 d after vaccination with IIV4 and resolved without sequelae. In conclusion, in children aged 3-8 y who received one dose or 2 doses 28 d apart, IIV4 had an acceptable safety profile, was as immunogenic as IIV3 for the shared strains, and had superior immunogenicity for the additional B strain.
Keywords: children; haemagglutination inhibition; immunogenicity; phase III clinical trial; quadrivalent inactivated influenza vaccine; safety.
Figures
References
-
- McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol 2004; 78:12817-28; PMID:15542634; http://dx.doi.org/ 10.1128/JVI.78.23.12817-12828.2004 - DOI - PMC - PubMed
-
- Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59-68; PMID:2309452; http://dx.doi.org/ 10.1016/0042-6822(90)90186-U - DOI - PubMed
-
- Centers for Disease Control Past Weekly Surveillance Reports, 2014. Available from: http://www.cdc.gov/flu/weekly/pastreports.htm
-
- Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine 2013; 31:5572-8; PMID:24016810; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.069 - DOI - PubMed
-
- Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, Santos J, Montalban MC, de Looze FJ, Eizenberg P, Hall S, Dupuy M, Hutagalung Y, et al.. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: A randomized, controlled, phase III trial. Vaccine 2015; 33:2485-92; PMID:25843270; http://dx.doi.org/ 10.1016/j.vaccine.2015.03.065 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
