Dental cell sheet biomimetic tooth bud model
- PMID: 27565550
- PMCID: PMC5025039
- DOI: 10.1016/j.biomaterials.2016.08.024
Dental cell sheet biomimetic tooth bud model
Abstract
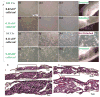
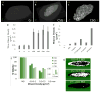
Tissue engineering and regenerative medicine technologies offer promising therapies for both medicine and dentistry. Our long-term goal is to create functional biomimetic tooth buds for eventual tooth replacement in humans. Here, our objective was to create a biomimetic 3D tooth bud model consisting of dental epithelial (DE) - dental mesenchymal (DM) cell sheets (CSs) combined with biomimetic enamel organ and pulp organ layers created using GelMA hydrogels. Pig DE or DM cells seeded on temperature-responsive plates at various cell densities (0.02, 0.114 and 0.228 cells 10(6)/cm(2)) and cultured for 7, 14 and 21 days were used to generate DE and DM cell sheets, respectively. Dental CSs were combined with GelMA encapsulated DE and DM cell layers to form bioengineered 3D tooth buds. Biomimetic 3D tooth bud constructs were cultured in vitro, or implanted in vivo for 3 weeks. Analyses were performed using micro-CT, H&E staining, polarized light (Pol) microscopy, immunofluorescent (IF) and immunohistochemical (IHC) analyses. H&E, IHC and IF analyses showed that in vitro cultured multilayered DE-DM CSs expressed appropriate tooth marker expression patterns including SHH, BMP2, RUNX2, tenascin and syndecan, which normally direct DE-DM interactions, DM cell condensation, and dental cell differentiation. In vivo implanted 3D tooth bud constructs exhibited mineralized tissue formation of specified size and shape, and SHH, BMP2 and RUNX2and dental cell differentiation marker expression. We propose our biomimetic 3D tooth buds as models to study optimized DE-DM cell interactions leading to functional biomimetic replacement tooth formation.
Keywords: Biomaterials; Dental stem cells; Regenerative medicine; Tissue engineering; Tooth development.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures








Similar articles
-
Developing a biomimetic tooth bud model.J Tissue Eng Regen Med. 2017 Dec;11(12):3326-3336. doi: 10.1002/term.2246. Epub 2017 Jan 8. J Tissue Eng Regen Med. 2017. PMID: 28066993 Free PMC article.
-
Decellularized Tooth Bud Scaffolds for Tooth Regeneration.J Dent Res. 2017 May;96(5):516-523. doi: 10.1177/0022034516689082. Epub 2017 Jan 24. J Dent Res. 2017. PMID: 28118552 Free PMC article.
-
Bioengineered Tooth Buds Exhibit Features of Natural Tooth Buds.J Dent Res. 2018 Sep;97(10):1144-1151. doi: 10.1177/0022034518779075. Epub 2018 Jun 7. J Dent Res. 2018. PMID: 29879370 Free PMC article.
-
Tooth Repair and Regeneration: Potential of Dental Stem Cells.Trends Mol Med. 2021 May;27(5):501-511. doi: 10.1016/j.molmed.2021.02.005. Epub 2021 Mar 26. Trends Mol Med. 2021. PMID: 33781688 Free PMC article. Review.
-
Bioengineered teeth from tooth bud cells.Dent Clin North Am. 2006 Apr;50(2):191-203, viii. doi: 10.1016/j.cden.2005.11.005. Dent Clin North Am. 2006. PMID: 16530057 Review.
Cited by
-
Advanced in Vitro Experimental Models for Tissue Engineering-based Reconstruction of a 3D Dentin/pulp Complex: a Literature Review.Stem Cell Rev Rep. 2021 Jun;17(3):785-802. doi: 10.1007/s12015-020-10069-8. Epub 2020 Nov 3. Stem Cell Rev Rep. 2021. PMID: 33145672 Review.
-
Synthetic materials in craniofacial regenerative medicine: A comprehensive overview.Front Bioeng Biotechnol. 2022 Nov 9;10:987195. doi: 10.3389/fbioe.2022.987195. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36440445 Free PMC article. Review.
-
3D-bioprinted Recombination Structure of Hertwig's Epithelial Root Sheath Cells and Dental Papilla Cells for Alveolar Bone Regeneration.Int J Bioprint. 2022 Jun 10;8(3):512. doi: 10.18063/ijb.v8i3.512. eCollection 2022. Int J Bioprint. 2022. PMID: 36105141 Free PMC article.
-
Hard Dental Tissues Regeneration-Approaches and Challenges.Materials (Basel). 2021 May 14;14(10):2558. doi: 10.3390/ma14102558. Materials (Basel). 2021. PMID: 34069265 Free PMC article. Review.
-
Viability of Quercetin-Induced Dental Pulp Stem Cells in Forming Living Cellular Constructs for Soft Tissue Augmentation.J Pers Med. 2021 May 18;11(5):430. doi: 10.3390/jpm11050430. J Pers Med. 2021. PMID: 34070084 Free PMC article.
References
-
- Inui A, Itamoto K, Takuma T, Tsutsumi H, Tanigawa M, Hayasaki M, et al. Age-related changes of bone mineral density and microarchitecture in miniature pigs. Journal of Veterinary Medical Science. 2004;66(6):599–609. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

