AMD-like retinopathy associated with intravenous iron
- PMID: 27565570
- PMCID: PMC5045814
- DOI: 10.1016/j.exer.2016.08.008
AMD-like retinopathy associated with intravenous iron
Abstract
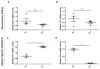
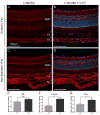
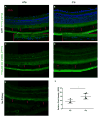
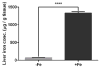
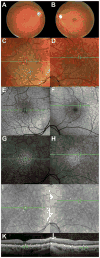
Iron accumulation in the retina is associated with the development of age-related macular degeneration (AMD). IV iron is a common method to treat iron deficiency anemia in adults, and its retinal manifestations have not hitherto been identified. To assess whether IV iron formulations can be retina-toxic, we generated a mouse model for iron-induced retinal damage. Male C57BL/6J mice were randomized into groups receiving IV iron-sucrose (+Fe) or 30% sucrose (-Fe). Iron levels in neurosensory retina (NSR), retinal pigment epithelium (RPE), and choroid were assessed using immunofluorescence, quantitative PCR, and the Perls' iron stain. Iron levels were most increased in the RPE and choroid while levels in the NSR did not differ significantly in +Fe mice compared to controls. Eyes from +Fe mice shared histological features with AMD, including Bruch's membrane (BrM) thickening with complement C3 deposition, as well as RPE hypertrophy and vacuolization. This focal degeneration correlated with areas of high choroidal iron levels. Ultrastructural analysis provided further detail of the RPE/photoreceptor outer segment vacuolization and Bruch's membrane thickening. Findings were correlated with a clinical case of a 43-year-old patient who developed numerous retinal drusen, the hallmark of AMD, within 11 months of IV iron therapy. Our results suggest that IV iron therapy may have the potential to induce or exacerbate a form of retinal degeneration. This retinal degeneration shares features with AMD, indicating the need for further study of AMD risk in patients receiving IV iron treatment.
Keywords: AMD; Iron; Macular degeneration; Oxidative stress; RPE.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No authors have conflicts of interest.
Figures



Similar articles
-
CD1 Mouse Retina Is Shielded From Iron Overload Caused by a High Iron Diet.Invest Ophthalmol Vis Sci. 2015 Aug;56(9):5344-52. doi: 10.1167/iovs.15-17026. Invest Ophthalmol Vis Sci. 2015. PMID: 26275132 Free PMC article.
-
Iron accumulation in Bruch's membrane and melanosomes of donor eyes with age-related macular degeneration.Exp Eye Res. 2015 Aug;137:39-49. doi: 10.1016/j.exer.2015.05.019. Epub 2015 May 28. Exp Eye Res. 2015. PMID: 26026877
-
Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD.Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2728-36. doi: 10.1167/iovs.07-1472. Epub 2008 Mar 7. Invest Ophthalmol Vis Sci. 2008. PMID: 18326691 Free PMC article.
-
Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD).Prog Retin Eye Res. 2010 May;29(3):169-90. doi: 10.1016/j.preteyeres.2010.02.002. Epub 2010 Mar 3. Prog Retin Eye Res. 2010. PMID: 20206286 Free PMC article. Review.
-
Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex.Mol Aspects Med. 2012 Aug;33(4):295-317. doi: 10.1016/j.mam.2012.04.005. Epub 2012 Apr 21. Mol Aspects Med. 2012. PMID: 22542780 Free PMC article. Review.
Cited by
-
Inflammatory adipose activates a nutritional immunity pathway leading to retinal dysfunction.Cell Rep. 2022 Jun 14;39(11):110942. doi: 10.1016/j.celrep.2022.110942. Cell Rep. 2022. PMID: 35705048 Free PMC article.
-
Release of Iron-Loaded Ferritin in Sodium Iodate-Induced Model of Age Related Macular Degeneration: An In-Vitro and In-Vivo Study.Antioxidants (Basel). 2021 Aug 5;10(8):1253. doi: 10.3390/antiox10081253. Antioxidants (Basel). 2021. PMID: 34439501 Free PMC article.
-
Ferroptosis: An Energetic Villain of Age-Related Macular Degeneration.Biomedicines. 2025 Apr 17;13(4):986. doi: 10.3390/biomedicines13040986. Biomedicines. 2025. PMID: 40299661 Free PMC article. Review.
-
Minimal effect of conditional ferroportin KO in the neural retina implicates ferrous iron in retinal iron overload and degeneration.Exp Eye Res. 2022 May;218:108988. doi: 10.1016/j.exer.2022.108988. Epub 2022 Feb 21. Exp Eye Res. 2022. PMID: 35202704 Free PMC article.
-
Retinal Degeneration and Alzheimer's Disease: An Evolving Link.Int J Mol Sci. 2020 Oct 2;21(19):7290. doi: 10.3390/ijms21197290. Int J Mol Sci. 2020. PMID: 33023198 Free PMC article. Review.
References
-
- Biesemeier A, Yoeruek E, Eibl O, Schraermeyer U. Iron accumulation in Bruch’s membrane and melanosomes of donor eyes with age-related macular degeneration. Exp Eye Res. 2015;137:39–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

