Nectrisine Biosynthesis Genes in Thelonectria discophora SANK 18292: Identification and Functional Analysis
- PMID: 27565616
- PMCID: PMC5066358
- DOI: 10.1128/AEM.01709-16
Nectrisine Biosynthesis Genes in Thelonectria discophora SANK 18292: Identification and Functional Analysis
Abstract
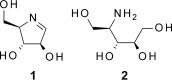
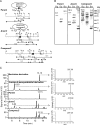
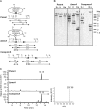
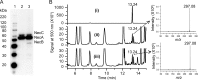
The fungus Thelonectria discophora SANK 18292 produces the iminosugar nectrisine, which has a nitrogen-containing heterocyclic 5-membered ring and acts as a glycosidase inhibitor. In our previous study, an oxidase (designated NecC) that converts 4-amino-4-deoxyarabinitol to nectrisine was purified from T. discophora cultures. However, the genes required for nectrisine biosynthesis remained unclear. In this study, the nectrisine biosynthetic gene cluster in T. discophora was identified from the contiguous genome sequence around the necC gene. Gene disruption and complementation studies and heterologous expression of the gene showed that necA, necB, and necC could be involved in nectrisine biosynthesis, during which amination, dephosphorylation, and oxidation occur. It was also demonstrated that nectrisine could be produced by recombinant Escherichia coli coexpressing the necA, necB, and necC genes. These findings provide the foundation to develop a bacterial production system for nectrisine or its intermediates through genetic engineering.
Importance: Iminosugars might have great therapeutic potential for treatment of many diseases. However, information on the genes for their biosynthesis is limited. In this study, we report the identification of genes required for biosynthesis of the iminosugar nectrisine in Thelonectria discophora SANK 18292, which was verified by disruption, complementation, and heterologous expression of the genes involved. We also demonstrate heterologous production of nectrisine by recombinant E. coli, toward developing an efficient production system for nectrisine or its intermediates through genetic engineering.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Biosynthesis of nectrisine in Thelonectria discophora SANK 18292.Phytochemistry. 2015 Aug;116:87-93. doi: 10.1016/j.phytochem.2015.03.011. Epub 2015 Apr 9. Phytochemistry. 2015. PMID: 25865736
-
Characterization of a novel oxidase from Thelonectria discophora SANK 18292 involved in nectrisine biosynthesis.AMB Express. 2016 Mar;6(1):6. doi: 10.1186/s13568-016-0176-1. Epub 2016 Jan 20. AMB Express. 2016. PMID: 26786316 Free PMC article.
-
De novo genome assembly and annotation of rice sheath rot fungus Sarocladium oryzae reveals genes involved in Helvolic acid and Cerulenin biosynthesis pathways.BMC Genomics. 2016 Mar 31;17:271. doi: 10.1186/s12864-016-2599-0. BMC Genomics. 2016. PMID: 27036298 Free PMC article.
-
Structure and synthesis of nectrisine, a new immunomodulator isolated from a fungus.Chem Pharm Bull (Tokyo). 1991 Nov;39(11):2807-12. doi: 10.1248/cpb.39.2807. Chem Pharm Bull (Tokyo). 1991. PMID: 1799935
-
Functional Analysis of a Gene Cluster from Chitinophaga pinensis Involved in Biosynthesis of the Pyrrolidine Azasugar DAB-1.J Nat Prod. 2019 Dec 27;82(12):3401-3409. doi: 10.1021/acs.jnatprod.9b00758. Epub 2019 Dec 3. J Nat Prod. 2019. PMID: 31793783
Cited by
-
Streptomyces lydicus M01 Regulates Soil Microbial Community and Alleviates Foliar Disease Caused by Alternaria alternata on Cucumbers.Front Microbiol. 2020 May 15;11:942. doi: 10.3389/fmicb.2020.00942. eCollection 2020. Front Microbiol. 2020. PMID: 32499771 Free PMC article.
-
Bacterial pathogen deploys the iminosugar glycosyrin to manipulate plant glycobiology.Science. 2025 Apr 18;388(6744):297-303. doi: 10.1126/science.adp2433. Epub 2025 Apr 17. Science. 2025. PMID: 40245141 Free PMC article.
-
Tin(iv) chloride mediated (3 + 2) annulation of trans-2-aroyl-3-styrylcyclopropane-1,1-dicarboxylates with nitriles: diastereoselective access to 5-vinyl-1-pyrroline derivatives.RSC Adv. 2021 Apr 21;11(25):14980-14985. doi: 10.1039/d1ra01194d. eCollection 2021 Apr 21. RSC Adv. 2021. PMID: 35424056 Free PMC article.
-
Metal-mediated synthesis of pyrrolines.RSC Adv. 2019 Feb 27;9(12):6804-6844. doi: 10.1039/c8ra10247c. eCollection 2019 Feb 22. RSC Adv. 2019. PMID: 35518475 Free PMC article. Review.
-
Bacterial pathogen deploys iminosugar galactosyrin to manipulate plant glycobiology.bioRxiv [Preprint]. 2025 Feb 14:2025.02.13.638044. doi: 10.1101/2025.02.13.638044. bioRxiv. 2025. Update in: Science. 2025 Apr 18;388(6744):297-303. doi: 10.1126/science.adp2433. PMID: 39990308 Free PMC article. Updated. Preprint.
References
-
- Compain P, Martin OR. 2007. Iminosugars: from synthesis to therapeutic applications. Wiley, Hoboken, NJ.
-
- Winchester BG. 2009. Iminosugars: from botanical curiosities to licensed drugs. Tetrahedron Asymmetry 20:645–651. doi:10.1016/j.tetasy.2009.02.048. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

