Orenia metallireducens sp. nov. Strain Z6, a Novel Metal-Reducing Member of the Phylum Firmicutes from the Deep Subsurface
- PMID: 27565620
- PMCID: PMC5066361
- DOI: 10.1128/AEM.02382-16
Orenia metallireducens sp. nov. Strain Z6, a Novel Metal-Reducing Member of the Phylum Firmicutes from the Deep Subsurface
Abstract
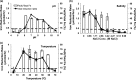
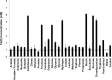
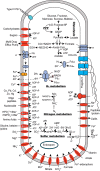
A novel halophilic and metal-reducing bacterium, Orenia metallireducens strain Z6, was isolated from briny groundwater extracted from a 2.02 km-deep borehole in the Illinois Basin, IL. This organism shared 96% 16S rRNA gene similarity with Orenia marismortui but demonstrated physiological properties previously unknown for this genus. In addition to exhibiting a fermentative metabolism typical of the genus Orenia, strain Z6 reduces various metal oxides [Fe(III), Mn(IV), Co(III), and Cr(VI)], using H2 as the electron donor. Strain Z6 actively reduced ferrihydrite over broad ranges of pH (6 to 9.6), salinity (0.4 to 3.5 M NaCl), and temperature (20 to 60°C). At pH 6.5, strain Z6 also reduced more crystalline iron oxides, such as lepidocrocite (γ-FeOOH), goethite (α-FeOOH), and hematite (α-Fe2O3). Analysis of X-ray absorption fine structure (XAFS) following Fe(III) reduction by strain Z6 revealed spectra from ferrous secondary mineral phases consistent with the precipitation of vivianite [Fe3(PO4)2] and siderite (FeCO3). The draft genome assembled for strain Z6 is 3.47 Mb in size and contains 3,269 protein-coding genes. Unlike the well-understood iron-reducing Shewanella and Geobacter species, this organism lacks the c-type cytochromes for typical Fe(III) reduction. Strain Z6 represents the first bacterial species in the genus Orenia (order Halanaerobiales) reported to reduce ferric iron minerals and other metal oxides. This microbe expands both the phylogenetic and physiological scopes of iron-reducing microorganisms known to inhabit the deep subsurface and suggests new mechanisms for microbial iron reduction. These distinctions from other Orenia spp. support the designation of strain Z6 as a new species, Orenia metallireducens sp. nov.
Importance: A novel iron-reducing species, Orenia metallireducens sp. nov., strain Z6, was isolated from groundwater collected from a geological formation located 2.02 km below land surface in the Illinois Basin, USA. Phylogenetic, physiologic, and genomic analyses of strain Z6 found it to have unique properties for iron reducers, including (i) active microbial iron-reducing capacity under broad ranges of temperatures (20 to 60°C), pHs (6 to 9.6), and salinities (0.4 to 3.5 M NaCl), (ii) lack of c-type cytochromes typically affiliated with iron reduction in Geobacter and Shewanella species, and (iii) being the only member of the Halanaerobiales capable of reducing crystalline goethite and hematite. This study expands the scope of phylogenetic affiliations, metabolic capacities, and catalytic mechanisms for iron-reducing microbes.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Controls on Iron Reduction and Biomineralization over Broad Environmental Conditions as Suggested by the Firmicutes Orenia metallireducens Strain Z6.Environ Sci Technol. 2020 Aug 18;54(16):10128-10140. doi: 10.1021/acs.est.0c03853. Epub 2020 Aug 6. Environ Sci Technol. 2020. PMID: 32693580
-
Geobacter pickeringii sp. nov., Geobacter argillaceus sp. nov. and Pelosinus fermentans gen. nov., sp. nov., isolated from subsurface kaolin lenses.Int J Syst Evol Microbiol. 2007 Jan;57(Pt 1):126-135. doi: 10.1099/ijs.0.64221-0. Int J Syst Evol Microbiol. 2007. PMID: 17220454
-
Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates.Int J Syst Evol Microbiol. 2005 Jul;55(Pt 4):1667-1674. doi: 10.1099/ijs.0.63417-0. Int J Syst Evol Microbiol. 2005. PMID: 16014499
-
Dissimilatory Fe(III) and Mn(IV) reduction.Adv Microb Physiol. 2004;49:219-86. doi: 10.1016/S0065-2911(04)49005-5. Adv Microb Physiol. 2004. PMID: 15518832 Review.
-
The electrifying physiology of Geobacter bacteria, 30 years on.Adv Microb Physiol. 2019;74:1-96. doi: 10.1016/bs.ampbs.2019.02.007. Epub 2019 May 15. Adv Microb Physiol. 2019. PMID: 31126529 Review.
Cited by
-
Enhanced bioelectrochemical degradation of Thiabendazole using biostimulated Tunisian hypersaline sediments: kinetics, efficiency, and microbial community shifts.Front Microbiol. 2025 Jan 6;15:1529841. doi: 10.3389/fmicb.2024.1529841. eCollection 2024. Front Microbiol. 2025. PMID: 39834368 Free PMC article.
-
Impact of salinity origin on microbial communities in saline springs within the Illinois Basin, USA.Environ Microbiol. 2022 Dec;24(12):6112-6127. doi: 10.1111/1462-2920.16241. Epub 2022 Nov 7. Environ Microbiol. 2022. PMID: 36222141 Free PMC article.
-
Microorganisms from deep-sea hydrothermal vents.Mar Life Sci Technol. 2021 Jan 22;3(2):204-230. doi: 10.1007/s42995-020-00086-4. eCollection 2021 May. Mar Life Sci Technol. 2021. PMID: 37073341 Free PMC article. Review.
-
Illegal dumping of oil and gas wastewater alters arid soil microbial communities.Appl Environ Microbiol. 2024 Feb 21;90(2):e0149023. doi: 10.1128/aem.01490-23. Epub 2024 Jan 31. Appl Environ Microbiol. 2024. PMID: 38294246 Free PMC article.
-
Impact of iron reduction on the metabolism of Clostridium acetobutylicum.Environ Microbiol. 2019 Oct;21(10):3548-3563. doi: 10.1111/1462-2920.14640. Epub 2019 May 16. Environ Microbiol. 2019. PMID: 31020759 Free PMC article.
References
-
- Heimann A, Johnson CM, Beard BL, Valley JW, Roden EE, Spicuzza MJ, Beukes NJ. 2010. Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in similar to 25 Ga marine environments. Earth Planet Sci Lett 294:8–18. doi: 10.1016/j.epsl.2010.02.015. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

