A Survival Strategy for Pseudomonas aeruginosa That Uses Exopolysaccharides To Sequester and Store Iron To Stimulate Psl-Dependent Biofilm Formation
- PMID: 27565622
- PMCID: PMC5066357
- DOI: 10.1128/AEM.01307-16
A Survival Strategy for Pseudomonas aeruginosa That Uses Exopolysaccharides To Sequester and Store Iron To Stimulate Psl-Dependent Biofilm Formation
Abstract
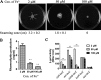
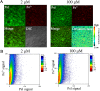
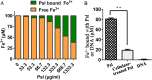
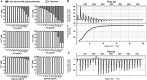
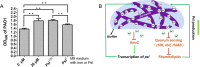
Exopolysaccharide Psl is a critical biofilm matrix component in Pseudomonas aeruginosa, which forms a fiber-like matrix to enmesh bacterial communities. Iron is important for P. aeruginosa biofilm development, yet it is not clearly understood how iron contributes to biofilm development. Here, we showed that iron promoted biofilm formation via elevating Psl production in P. aeruginosa The high level of iron stimulated the synthesis of Psl by reducing rhamnolipid biosynthesis and inhibiting the expression of AmrZ, a repressor of psl genes. Iron-stimulated Psl biosynthesis and biofilm formation held true in mucoid P. aeruginosa strains. Subsequent experiments indicated that iron bound with Psl in vitro and in biofilms, which suggested that Psl fibers functioned as an iron storage channel in P. aeruginosa biofilms. Moreover, among three matrix exopolysaccharides of P. aeruginosa, Psl is the only exopolysaccharide that can bind with both ferrous and ferric ion, yet with higher affinity for ferrous iron. Our data suggest a survival strategy of P. aeruginosa that uses exopolysaccharide to sequester and store iron to stimulate Psl-dependent biofilm formation.
Importance: Pseudomonas aeruginosa is an environmental microorganism which is also an opportunistic pathogen that can cause severe infections in immunocompromised individuals. It is the predominant airway pathogen causing morbidity and mortality in individuals affected by the genetic disease cystic fibrosis (CF). Increased airway iron and biofilm formation have been proposed to be the potential factors involved in the persistence of P. aeruginosa in CF patients. Here, we showed that a high level of iron enhanced the production of the key biofilm matrix exopolysaccharide Psl to stimulate Psl-dependent biofilm formation. Our results not only make the link between biofilm formation and iron concentration in CF, but also could guide the administration or use of iron chelators to interfere with biofilm formation in P. aeruginosa in CF patients. Furthermore, our data also imply a survival strategy of P. aeruginosa under high-iron environmental conditions.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


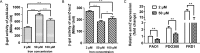
Similar articles
-
The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms.FEMS Immunol Med Microbiol. 2012 Jul;65(2):377-80. doi: 10.1111/j.1574-695X.2012.00934.x. Epub 2012 Mar 12. FEMS Immunol Med Microbiol. 2012. PMID: 22309106
-
Iron-regulated expression of alginate production, mucoid phenotype, and biofilm formation by Pseudomonas aeruginosa.mBio. 2014 Feb 4;5(1):e01010-13. doi: 10.1128/mBio.01010-13. mBio. 2014. PMID: 24496793 Free PMC article.
-
Coordination of swarming motility, biosurfactant synthesis, and biofilm matrix exopolysaccharide production in Pseudomonas aeruginosa.Appl Environ Microbiol. 2014 Nov;80(21):6724-32. doi: 10.1128/AEM.01237-14. Epub 2014 Aug 29. Appl Environ Microbiol. 2014. PMID: 25172852 Free PMC article.
-
Matrix exopolysaccharides; the sticky side of biofilm formation.FEMS Microbiol Lett. 2017 Jul 6;364(13):fnx120. doi: 10.1093/femsle/fnx120. FEMS Microbiol Lett. 2017. PMID: 28605431 Free PMC article. Review.
-
Regulation of Biofilm Exopolysaccharide Biosynthesis and Degradation in Pseudomonas aeruginosa.Annu Rev Microbiol. 2022 Sep 8;76:413-433. doi: 10.1146/annurev-micro-041320-111355. Epub 2022 Jun 2. Annu Rev Microbiol. 2022. PMID: 35655342 Review.
Cited by
-
Interbacterial Antagonism Mediated by a Released Polysaccharide.J Bacteriol. 2022 May 17;204(5):e0007622. doi: 10.1128/jb.00076-22. Epub 2022 Apr 21. J Bacteriol. 2022. PMID: 35446119 Free PMC article.
-
Luminescent Nanosensors for Ratiometric Monitoring of Three-Dimensional Oxygen Gradients in Laboratory and Clinical Pseudomonas aeruginosa Biofilms.Appl Environ Microbiol. 2019 Oct 1;85(20):e01116-19. doi: 10.1128/AEM.01116-19. Print 2019 Oct 15. Appl Environ Microbiol. 2019. PMID: 31420335 Free PMC article.
-
Strategies for Interfering With Bacterial Early Stage Biofilms.Front Microbiol. 2021 Jun 8;12:675843. doi: 10.3389/fmicb.2021.675843. eCollection 2021. Front Microbiol. 2021. PMID: 34168632 Free PMC article. Review.
-
Manganese Acts as an Environmental Inhibitor of Pseudomonas aeruginosa Biofilm Development by Inducing Dispersion and Modulating c-di-GMP and Exopolysaccharide Production via RbdA.J Bacteriol. 2023 Jun 27;205(6):e0000323. doi: 10.1128/jb.00003-23. Epub 2023 May 18. J Bacteriol. 2023. PMID: 37199658 Free PMC article.
-
Iron Homeostasis in Bacillus subtilis Requires Siderophore Production and Biofilm Formation.Appl Environ Microbiol. 2019 Jan 23;85(3):e02439-18. doi: 10.1128/AEM.02439-18. Print 2019 Feb 1. Appl Environ Microbiol. 2019. PMID: 30446551 Free PMC article.
References
-
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi:10.1038/35023079. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

