Adding a single low-dose of primaquine (0.25 mg/kg) to artemether-lumefantrine did not compromise treatment outcome of uncomplicated Plasmodium falciparum malaria in Tanzania: a randomized, single-blinded clinical trial
- PMID: 27565897
- PMCID: PMC5002101
- DOI: 10.1186/s12936-016-1430-3
Adding a single low-dose of primaquine (0.25 mg/kg) to artemether-lumefantrine did not compromise treatment outcome of uncomplicated Plasmodium falciparum malaria in Tanzania: a randomized, single-blinded clinical trial
Abstract
Background: The World Health Organization (WHO) recently recommended the addition of a single low-dose of the gametocytocidal drug primaquine (PQ) to artemisinin-based combination therapy (ACT) in low transmission settings as a component of pre-elimination or elimination programmes. However, it is unclear whether that influences the ACT cure rate. The study assessed treatment outcome of artemether-lumefantrine (AL) plus a single PQ dose (0.25 mg/kg) versus standard AL regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania.
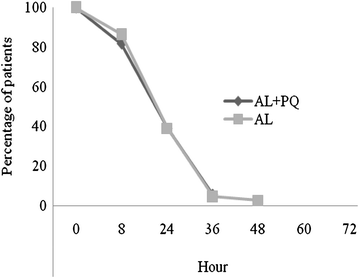
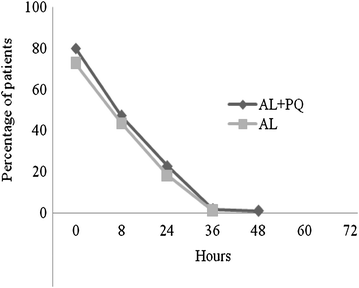
Methods: A randomized, single-blinded, clinical trial was conducted in Yombo, Bagamoyo district, Tanzania. Acute uncomplicated P. falciparum malaria patients aged ≥1 year, with the exception of pregnant and lactating women, were enrolled and treated with AL plus a single PQ dose (0.25 mg/kg) or AL alone under supervision. PQ was administered together with the first AL dose. Clinical and laboratory assessments were performed at 0, 8, 24, 36, 48, 60, and 72 h and on days 7, 14, 21, and 28. The primary end-point was a polymerase chain reaction (PCR)-adjusted adequate clinical and parasitological response (ACPR) on day 28. Secondary outcomes included: fever and asexual parasitaemia clearance, proportion of patients with PCR-determined parasitaemia on day 3, and proportion of patients with Pfmdr1 N86Y and Pfcrt K76T on days 0, 3 and day of recurrent infection.
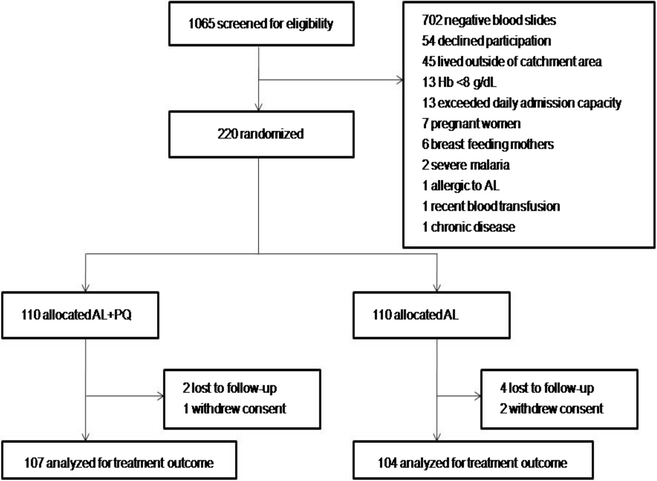
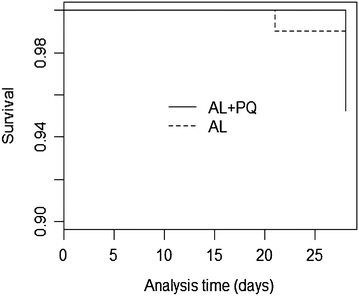
Results: Overall 220 patients were enrolled, 110 were allocated AL + PQ and AL, respectively. Parasite clearance by microscopy was fast, but PCR detectable parasitaemia on day 3 was 31/109 (28.4 %) and 29/108 (26.9 %) in patients treated with AL + PQ and AL, respectively (p = 0.79). Day 28 PCR-adjusted ACPR and re-infection rate was 105/105 (100 %) and 101/102 (99 %) (p = 0.31), and 5/107 (4.7 %) and 5/8 (4.8 %) (p = 0.95), in AL + PQ and AL arm, respectively. There was neither any statistically significant difference in the proportion of Pfmdr1 N86Y or Pfcrt K76T between treatment arms on days 0, 3 and day of recurrent infection, nor within treatment arms between days 0 and 3 or day 0 and day of recurrent infection.
Conclusion: The new WHO recommendation of adding a single low-dose of PQ to AL did not compromise treatment outcome of uncomplicated P. falciparum malaria in Tanzania. Trial registration number NCT02090036.
Keywords: Artemether-lumefantrine; Cure rate; Plasmodium falciparum malaria; Primaquine.
Figures
References
-
- WHO. World Malaria Report 2010. Geneva, World Health Organization, 2010.
-
- WHO. Evidence Review Group: The safety and effectiveness of single dose primaquine as a P. falciparum gametocytocide. Geneva, World Health Organization, 2012.
-
- WHO. Guidelines for the treatment of malaria. 3rd ed. Geneva, World Health Organization, 2015. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical