Quantification of PD-L1 and PD-1 expression on tumor and immune cells in non-small cell lung cancer (NSCLC) using non-enzymatic tissue dissociation and flow cytometry
- PMID: 27565980
- PMCID: PMC11028699
- DOI: 10.1007/s00262-016-1889-3
Quantification of PD-L1 and PD-1 expression on tumor and immune cells in non-small cell lung cancer (NSCLC) using non-enzymatic tissue dissociation and flow cytometry
Abstract
Objective: We report a truly quantitative technology for PD-L1 expression in non-small cell lung cancer (NSCLC). In addition, we present a non-enzymatic technology that creates a cell suspension from fresh tumor tissue so that either fine-needle aspiration (FNA) or fresh tissue can be used in this assay.
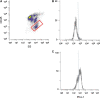
Methods: Non-enzymatic tissue homogenization (IncellPREP; IncellDx, Menlo Park, California) was performed on 4-mm punch biopsies. An FNA was taken from the same tumor to create matched sample sets. Cells were labeled with antibodies directed against CD45, PD-1, and PD-L1 and then stained with DAPI to identify intact, single cells, and to analyze cell cycle.
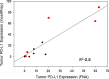
Results: Comparing the IncellPREP homogenization and FNA demonstrated a strong correlation (r 2 - 0.8) for expression of PD-L1. We compared PD-L1 expression by flow cytometry using a 1 % cutoff for positivity in the tumor cell population and a 1 % cutoff of cells with at least 1+ intensity in immunohistochemically stained tissue sections as positive. Ten of 12 lung tumor samples were concordant while 2 were discordant. PD-L1 expression by flow cytometry varied widely (1.2-89.4 %) even in the positive concordant cases. In addition, PD-L1 expression in the aneuploid tumor population did not necessarily agree with the expression in the diploid tumor population. Fine, unequivocal quantification of PD-L1 on tumor and immune cells in NSCLC may allow for better prediction of response to therapies. The present study also offers a technology that can create a universal sample type from either FNA or fresh tissue.
Keywords: Cell cycle; Immune cells; Non-small cell lung cancer; PD-1; PD-L1.
Conflict of interest statement
Amanda Chargin, Rian Morgan, Keith Shults, and Bruce K. Patterson are employees of IncellDx, Inc., Ellen Tsay was an uncompensated intern of IncellDx, Inc., Uma Sundram and Navneet Ratti declare that they have no conflict of interest.
Figures

Similar articles
-
Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations.Cancer Cytopathol. 2018 May;126(5):342-352. doi: 10.1002/cncy.21987. Epub 2018 Mar 2. Cancer Cytopathol. 2018. PMID: 29499101
-
PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens.Cancer Cytopathol. 2017 Dec;125(12):896-907. doi: 10.1002/cncy.21937. Epub 2017 Oct 12. Cancer Cytopathol. 2017. PMID: 29024471
-
Performance of Ventana SP263 PD-L1 assay in endobronchial ultrasound guided-fine-needle aspiration derived non-small-cell lung carcinoma samples.Diagn Cytopathol. 2021 Mar;49(3):355-362. doi: 10.1002/dc.24654. Epub 2020 Nov 3. Diagn Cytopathol. 2021. PMID: 33142053
-
The Significance of the PD-L1 Expression in Non-Small-Cell Lung Cancer: Trenchant Double Swords as Predictive and Prognostic Markers.Clin Lung Cancer. 2018 Mar;19(2):120-129. doi: 10.1016/j.cllc.2017.10.014. Epub 2017 Oct 28. Clin Lung Cancer. 2018. PMID: 29153898 Review.
-
PD-L1 testing for lung cancer in the UK: recognizing the challenges for implementation.Histopathology. 2016 Aug;69(2):177-86. doi: 10.1111/his.12996. Epub 2016 Jun 30. Histopathology. 2016. PMID: 27196116 Review.
Cited by
-
Atezolizumab in non-small cell lung cancer: the era of precision immuno-oncology.Ann Transl Med. 2017 Jun;5(12):265. doi: 10.21037/atm.2017.03.89. Ann Transl Med. 2017. PMID: 28706933 Free PMC article. No abstract available.
-
Immunological effect of local ablation combined with immunotherapy on solid malignancies.Chin J Cancer. 2017 Jun 7;36(1):49. doi: 10.1186/s40880-017-0216-5. Chin J Cancer. 2017. PMID: 28592286 Free PMC article. Review.
-
Atezolizumab in advanced non-small cell lung cancer.J Thorac Dis. 2017 Oct;9(10):3603-3606. doi: 10.21037/jtd.2017.09.73. J Thorac Dis. 2017. PMID: 29268352 Free PMC article. No abstract available.
-
Flow cytometry detection of cell type-specific expression of programmed death receptor ligand-1 (PD-L1) in colorectal cancer specimens.Heliyon. 2020 Dec 31;7(1):e05880. doi: 10.1016/j.heliyon.2020.e05880. eCollection 2021 Jan. Heliyon. 2020. PMID: 33458446 Free PMC article.
-
Neutrophil extracellular traps (NETs) reduce the diffusion of doxorubicin which may attenuate its ability to induce apoptosis of ovarian cancer cells.Heliyon. 2022 Jun 15;8(6):e09730. doi: 10.1016/j.heliyon.2022.e09730. eCollection 2022 Jun. Heliyon. 2022. PMID: 35756123 Free PMC article.
References
-
- Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

