Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus
- PMID: 27566110
- PMCID: PMC5002192
- DOI: 10.1186/s12866-016-0814-4
Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus
Abstract
Background: Globally, staphylococci have developed resistance to many antibiotics. New approaches to chemotherapy are needed and one such approach could be to use plant derived actives with conventional antibiotics in a synergestic way. The purpose of this study was to isolate α-mangostin from the mangosteen (Garcinia mangostana L.; GML) and investigate antibacterial activity and mechanisms of action when used singly and when combined with oxacillin against oxacillin-resistant Staphylococcus saprophyticus (ORSS) strains. The isolated α-mangostin was confirmed by HPLC chromatogram and NMR spectroscopy. The minimum inhibitory concentration (MIC), checkerboard and killing curve were determined. The modes of action of these compounds were also investigated by enzyme assay, transmission electron microscopy (TEM), confocal microscopic images, and cytoplasmic membrane (CM) permeabilization studies.
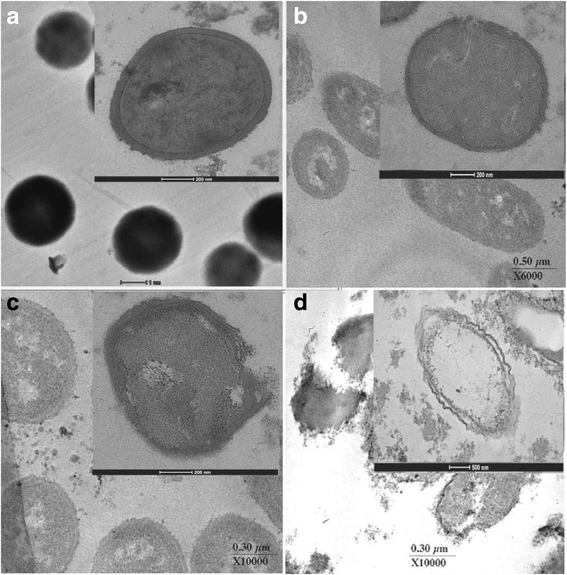
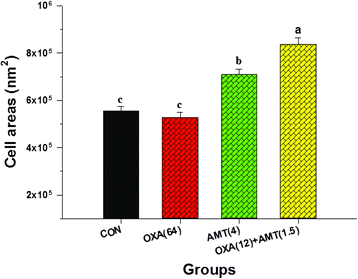
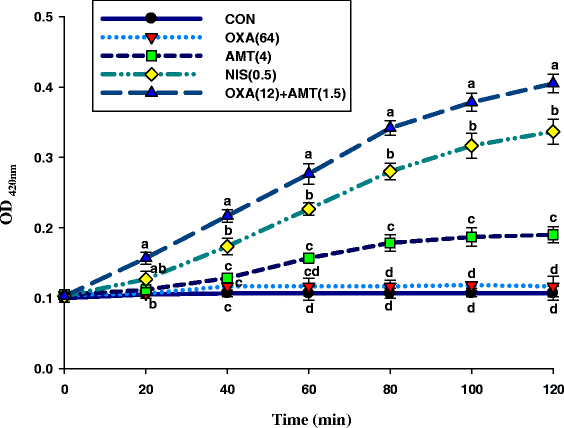
Results: The MICs of isolated α-mangostin and oxacillin against these strains were 8 and 128 μg/ml, respectively. Checkerboard assays showed the synergistic activity of isolated α-mangostin (2 μg/ml) plus oxacillin (16 μg/ml) at a fractional inhibitory concentration index (FICI) of 0.37. The kill curve assay confirmed that the viability of oxacillin-resistant Staphylococcus saprophyticus DMST 27055 (ORSS-27055) was dramatically reduced after exposure to isolated α-mangostin (2 μg/ml) plus oxacillin (16 μg/ml). Enzyme assays demonstrated that isolated α-mangostin had an inhibitory activity against β-lactamase in a dose-dependent manner. TEM results clearly showed that these ORSS-27055 cells treated with this combination caused peptidoglycan and cytoplasmic membrane damage, irregular cell shapes and average cell areas were significantly larger than the control. Clearly, confocal microscopic images confirmed that this combination caused considerable peptidoglycan damage and DNA leakage. In addition, the CM permeability of ORSS-27055 was also increased by this combination of actives.
Conclusions: These findings provide evidence that isolated α-mangostin alone has not only some activity but also shows the synergistic activity with oxacillin against ORSS-27055. The chromone and isoprenyl structures could play a significant role in its action. This synergistic activity may involve three mechanisms of action. Firstly, potential effects of cytoplasmic membrane disruption and increases permeability. Secondly, inhibit β-lactamase activity. Finally, also damage to the peptidoglycan structure. We proposes the potential to develop a novel adjunct phytopharmaceutical to oxacillin for the treatment of ORSS. Future studies require clinical trials to establish if the synergy reported can be translated to animals and humans.
Keywords: Garcinia mangostana; Mechanism of action; Oxacillin; Oxacillin-resistant S. saprophyticus; Synergistic activity; α-mangostin.
Figures







References
-
- Rukachaisirikul V, Arunpanichlert J, Sukpondma Y, Phongpaichit S, Sakayaroj J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron. 2009;65:10590–5. doi: 10.1016/j.tet.2009.10.084. - DOI
-
- Higashide M, Kuroda M, Omura CT, Kumano M, Ohkawa S, Ichimura S, et al. Methicillin-resistant Staphylococcus saprophyticus isolates carrying staphylococcal cassette chromosome mec have emerged in urogenital tract infections. Antimicrob Agents Chemother. 2008;52:2061–8. doi: 10.1128/AAC.01150-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

