A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma
- PMID: 27566443
- PMCID: PMC5035791
- DOI: 10.1093/annonc/mdw287
A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma
Abstract
Background: Recurrent/metastatic adenoid cystic carcinoma (ACC) is an incurable disease with no standard treatments. The majority of ACCs express the oncogenic transcription factor MYB (also c-myb), often in the context of a MYB gene rearrangement. This phase II trial of the tyrosine kinase inhibitor (TKI) axitinib (Pfizer) tested the hypothesis that targeting pathways activated by MYB can be therapeutically effective for ACC.
Patients and methods: This is a minimax two-stage, phase II trial that enrolled patients with incurable ACC of any primary site. Progressive or symptomatic disease was required. Patients were treated with axitinib 5 mg oral twice daily; dose escalation was allowed. The primary end point was best overall response (BOR). An exploratory analysis correlating biomarkers to drug benefit was conducted, including next-generation sequencing (NGS) in 11 patients.
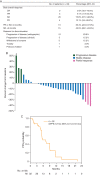
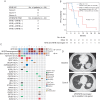
Results: Thirty-three patients were registered and evaluable for response. Fifteen patients had the axitinib dose increased. Tumor shrinkage was achieved in 22 (66.7%); 3 (9.1%) had confirmed partial responses. Twenty-five (75.8%) patients had stable disease, 10 of whom had disease stability for >6 months. The median progression-free survival (PFS) was 5.7 months (range 0.92-21.8 months). Grade 3 axitinib-related toxicities included hypertension, oral pain and fatigue. A trend toward superior PFS was noted with the MYB/NFIB rearrangement, although this was not statistically significant. NGS revealed three tumors with 4q12 amplification, producing increased copies of axitinib-targeted genes PDGFR/KDR/KIT. Two 4q12 amplified patients achieved stable disease for >6 months, including one with significant tumor reduction and the longest PFS on study (21.8 months).
Conclusions: Although the primary end point was not met, axitinib exhibited clinical activity with tumor shrinkage achieved in the majority of patients with progressive disease before trial enrollment. Analysis of MYB biomarkers and genomic profiling suggests the hypothesis that 4q12 amplified ACCs are a disease subset that benefit from TKI therapy.
Keywords: MYB; adenoid cystic carcinoma; axitinib.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett 2000; 154: 107–111. - PubMed
-
- Bernheim A, Toujani S, Saulnier P et al. . High-resolution array comparative genomic hybridization analysis of human bronchial and salivary adenoid cystic carcinoma. Lab Invest 2008; 88: 464–473. - PubMed
-
- Vekony H, Ylstra B, Wilting SM et al. . DNA copy number gains at loci of growth factors and their receptors in salivary gland adenoid cystic carcinoma. Clin Cancer Res 2007; 13: 3133–3139. - PubMed
-
- Rugo HS, Herbst RS, Liu G et al. . Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 2005; 23: 5474–5483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

