Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: A meta-analysis
- PMID: 27566453
- PMCID: PMC5568628
- DOI: 10.1016/j.jad.2016.08.014
Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: A meta-analysis
Abstract
Introduction: Major Depression Disorder (MDD) is common among mothers of young children. However, its detection remains low in primary-care and community-based settings in part due to the uncertainty regarding the validity of existing case-finding instruments. We conducted meta-analyses to estimate the diagnostic validity of commonly used maternal MDD case finding instruments in the United States.
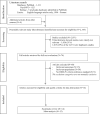
Methods: We systematically searched three electronic bibliographic databases PubMed, PsycINFO, and EMBASE from 1994 to 2015 to identify relevant published literature. Study eligibility and quality were evaluated using the Standards for the Reporting of Diagnostic Accuracy studies and Quality Assessment of Diagnostic Accuracy Studies guidelines, respectively. Pooled sensitivity and specificity of case-finding instruments were generated using Bayesian hierarchical summary receiver operating models.
Results: Overall, 1130 articles were retrieved and 74 articles were selected for full-text review. Twelve articles examining six maternal MDD case-finding instruments met the eligibility criteria and were included in our meta-analyses. Pooled sensitivity and specificity estimates were highest for the BDI-II (91%; 95% Bayesian Credible Interval (BCI): 68%; 99% and 89%; 95% BCI: 62%; 98% respectively) and EPDS10 (74%; 95% BCI: 46%; 91% and 97%; 95% BCI: 84%; 99% respectively) during the antepartum and postpartum periods respectively.
Limitation: No meta-regression was conducted to examine the impact of study-level characteristics on the results.
Discussion: Diagnostic performance varied among instruments and between peripartum periods. These findings suggest the need for a judicious selection of maternal MDD case-finding instruments depending on the study population and target periods of assessment.
Keywords: Bayesian meta-analysis; Case-finding instrument; Diagnostic performance; Major depression disorder; Misclassification error.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
None of the contributing authors and I have any conflict of interest in this subject or any financial interest. The opinions expressed are those of the authors and do not necessarily reflect those of the Oklahoma University Health Sciences Center.
Figures





Similar articles
-
Are we validly assessing major depression disorder risk and associated factors among mothers of young children? A cross-sectional study involving home visitation programs.PLoS One. 2019 Jan 7;14(1):e0209735. doi: 10.1371/journal.pone.0209735. eCollection 2019. PLoS One. 2019. PMID: 30615650 Free PMC article.
-
Diagnostic performance of major depression disorder case-finding instruments used among mothers of young children in the United States: A systematic review.J Affect Disord. 2016 Sep 1;201:185-93. doi: 10.1016/j.jad.2016.05.015. Epub 2016 May 13. J Affect Disord. 2016. PMID: 27240311 Free PMC article.
-
Impact of misclassification error in the estimation of maternal major depression disorder prevalence in home visitation programs.Psychiatry Res. 2018 Mar;261:80-87. doi: 10.1016/j.psychres.2017.12.047. Epub 2017 Dec 20. Psychiatry Res. 2018. PMID: 29289025
-
Validity of LIDAS (LIfetime Depression Assessment Self-report): a self-report online assessment of lifetime major depressive disorder.Psychol Med. 2017 Jan;47(2):279-289. doi: 10.1017/S0033291716002312. Epub 2016 Oct 5. Psychol Med. 2017. PMID: 27702414
-
Diagnostic Test Accuracy of the Beck Depression Inventory for Detecting Major Depression in Adolescents: A Systematic Review and Meta-Analysis.Clin Nurs Res. 2022 Nov;31(8):1481-1490. doi: 10.1177/10547738211065105. Epub 2021 Dec 27. Clin Nurs Res. 2022. PMID: 34961346
Cited by
-
Maternal major depression disorder misclassification errors: Remedies for valid individual- and population-level inference.Brain Behav. 2022 Jun;12(6):e2614. doi: 10.1002/brb3.2614. Epub 2022 May 19. Brain Behav. 2022. PMID: 35587518 Free PMC article.
-
Family physicians perceived role in perinatal mental health: an integrative review.BMC Fam Pract. 2018 Sep 8;19(1):154. doi: 10.1186/s12875-018-0843-1. BMC Fam Pract. 2018. PMID: 30193572 Free PMC article. Review.
-
Screening for perinatal depression with the Patient Health Questionnaire depression scale (PHQ-9): A systematic review and meta-analysis.Gen Hosp Psychiatry. 2021 Jan-Feb;68:74-82. doi: 10.1016/j.genhosppsych.2020.12.007. Epub 2020 Dec 21. Gen Hosp Psychiatry. 2021. PMID: 33360526 Free PMC article.
-
Satisfaction and Perceived Effectiveness on Herbal Decoctions for Postpartum Care: a cross-sectional survey of mother's experience.J Pharmacopuncture. 2023 Jun 30;26(2):175-183. doi: 10.3831/KPI.2023.26.2.175. J Pharmacopuncture. 2023. PMID: 37405116 Free PMC article.
-
Are we validly assessing major depression disorder risk and associated factors among mothers of young children? A cross-sectional study involving home visitation programs.PLoS One. 2019 Jan 7;14(1):e0209735. doi: 10.1371/journal.pone.0209735. eCollection 2019. PLoS One. 2019. PMID: 30615650 Free PMC article.
References
-
- Agency for Healthcare Research and Quality (AHRQ) Efficacy and Safety of Screening for Postpartum Depression. [accessed 11.01.14];Comparative Effectiveness Review. 106 Contract No. 290-2007-10066-I. 〈 http://www.ncbi.nlm.nih.gov/books/NBK137724〉.
-
- Beck CT, Gable RK. Comparative analysis of the performance of the post-partum depression screening scale with two other depression instruments. Nurs. Res. 2001;50(4):242–250. - PubMed
-
- Beck CT, Gable RK. Screening performance of the postpartum depression screening scale – Spanish version. J. Transcult. Nurs. 2005;16(4):331–338. - PubMed
-
- Bernatsky S, Joseph L, Belisle P, et al. Bayesian modelling of imperfect ascertainment methods in cancer studies. Stat. Med. 2005;24(15):2365–2379. - PubMed
-
- Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann. Intern. Med. 2003;138(1):W1–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

