Oncogenic STAT5 signaling promotes oxidative stress in chronic myeloid leukemia cells by repressing antioxidant defenses
- PMID: 27566554
- PMCID: PMC5522035
- DOI: 10.18632/oncotarget.11480
Oncogenic STAT5 signaling promotes oxidative stress in chronic myeloid leukemia cells by repressing antioxidant defenses
Abstract
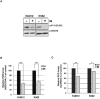
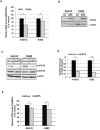
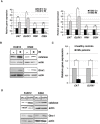
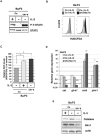
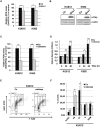
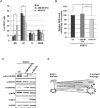
STAT5 transcription factors are frequently activated in hematopoietic neoplasms and are targets of various tyrosine kinase oncogenes. Evidences for a crosstalk between STAT5 and reactive oxygen species (ROS) metabolism have recently emerged but mechanisms involved in STAT5-mediated regulation of ROS still remain elusive. We demonstrate that sustained activation of STAT5 induced by Bcr-Abl in chronic myeloid leukemia (CML) cells promotes ROS production by repressing expression of two antioxidant enzymes, catalase and glutaredoxin-1(Glrx1). Downregulation of catalase and Glrx1 expression was also observed in primary cells from CML patients. Catalase was shown not only to reduce ROS levels but also, to induce quiescence in Bcr-Abl-positive leukemia cells. Furthermore, reduction of STAT5 phosphorylation and upregulation of catalase and Glrx1 were also evidenced in leukemia cells co-cultured with bone marrow stromal cells to mimic a leukemic niche. This caused downregulation of ROS levels and enhancement of leukemic cell quiescence. These data support a role of persistent STAT5 signaling in the regulation of ROS production in myeloid leukemias and highlight the repression of antioxidant defenses as an important regulatory mechanism.
Keywords: Bcr-Abl; STAT5; antioxidants; chronic myeloid leukemia; oxidative stress.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Bunting KD. STAT5 signaling in normal and pathologic hematopoiesis. Front Biosci. 2007;12:2807–20. - PubMed
-
- Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. - PubMed
-
- Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Müller C, Grüning W, Kratz-Albers K, Serve S, Steur C, Büchner T, Kienast J, Kanakura Y, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–14. - PubMed
-
- Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lannerée S, Kenner L, Kerenyi M, Yahiaoui S, Gouilleux-Gruart V, Gondry J, Bénit L, Dusanter-Fourt I, et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood. 2008;112:2463–73. - PMC - PubMed
-
- Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

