Efficacy and Safety of Brexpiprazole (OPC-34712) as Maintenance Treatment in Adults with Schizophrenia: a Randomized, Double-Blind, Placebo-Controlled Study
- PMID: 27566723
- PMCID: PMC5412583
- DOI: 10.1093/ijnp/pyw076
Efficacy and Safety of Brexpiprazole (OPC-34712) as Maintenance Treatment in Adults with Schizophrenia: a Randomized, Double-Blind, Placebo-Controlled Study
Abstract
Background: Brexpiprazole has previously demonstrated efficacy in acute schizophrenia trials. The objective of this trial was to assess the efficacy, safety, and tolerability of maintenance treatment with brexpiprazole in adults with schizophrenia.
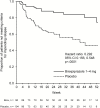
Methods: Patients with an acute exacerbation of psychotic symptoms were converted to brexpiprazole (1-4mg/d) over 1 to 4 weeks and entered a single-blind stabilization phase. Those patients who met stability criteria for 12 weeks were randomized 1:1 to double-blind maintenance treatment with either brexpiprazole (at their stabilization dose) or placebo for up to 52 weeks. The primary efficacy endpoint was the time from randomization to impending relapse. Safety and tolerability were also assessed.
Results: A total of 524 patients were enrolled, 202 of whom were stabilized on brexpiprazole and randomized to brexpiprazole (n=97) or placebo (n=105). Efficacy was demonstrated at a prespecified interim analysis (conducted after 45 events), and so the trial was terminated early. The final analysis showed that time to impending relapse was statistically significantly delayed with brexpiprazole treatment compared with placebo (P<.0001, log-rank test). The hazard ratio for the final analysis was 0.292 (95% confidence interval: 0.156, 0.548); mean dose at last visit, 3.6mg. The proportion of patients meeting the criteria for impending relapse was 13.5% with brexpiprazole and 38.5% with placebo (P<.0001). During the maintenance phase, the incidence of adverse events was comparable to placebo.
Conclusions: or patients with schizophrenia already stabilized on brexpiprazole, maintenance treatment with brexpiprazole was efficacious, with a favorable safety profile.
Trial registration: ClinicalTrials.gov NCT01668797.
Keywords: brexpiprazole; maintenance; relapse; schizophrenia; stabilization.
© The Author 2016. Published by Oxford University Press on behalf of CINP.
Figures

References
-
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th ed., text revision (DSM-IV-TR). Arlington, VA: American Psychiatric Association.
-
- Barnes TR. (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676. - PubMed
-
- Burns T, Patrick D. (2007) Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand 116:403–418. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

