Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy
- PMID: 27566742
- PMCID: PMC5109941
- DOI: 10.1212/WNL.0000000000003217
Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy
Abstract
Objective: To assess safety and efficacy of deflazacort (DFZ) and prednisone (PRED) vs placebo in Duchenne muscular dystrophy (DMD).
Methods: This phase III, double-blind, randomized, placebo-controlled, multicenter study evaluated muscle strength among 196 boys aged 5-15 years with DMD during a 52-week period. In phase 1, participants were randomly assigned to receive treatment with DFZ 0.9 mg/kg/d, DFZ 1.2 mg/kg/d, PRED 0.75 mg/kg/d, or placebo for 12 weeks. In phase 2, placebo participants were randomly assigned to 1 of the 3 active treatment groups. Participants originally assigned to an active treatment continued that treatment for an additional 40 weeks. The primary efficacy endpoint was average change in muscle strength from baseline to week 12 compared with placebo. The study was completed in 1995.
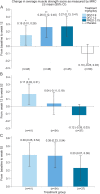
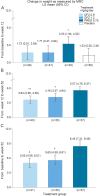
Results: All treatment groups (DFZ 0.9 mg/kg/d, DFZ 1.2 mg/kg/d, and PRED 0.75 mg/kg/d) demonstrated significant improvement in muscle strength compared with placebo at 12 weeks. Participants taking PRED had significantly more weight gain than placebo or both doses of DFZ at 12 weeks; at 52 weeks, participants taking PRED had significantly more weight gain than both DFZ doses. The most frequent adverse events in all 3 active treatment arms were Cushingoid appearance, erythema, hirsutism, increased weight, headache, and nasopharyngitis.
Conclusions: After 12 weeks of treatment, PRED and both doses of DFZ improved muscle strength compared with placebo. Deflazacort was associated with less weight gain than PRED.
Classification of evidence: This study provides Class I evidence that for boys with DMD, daily use of either DFZ and PRED is effective in preserving muscle strength over a 12-week period.
© 2016 American Academy of Neurology.
Figures

References
-
- Mendell JR, Shilling C, Leslie ND, et al. . Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012;71:304–313. - PubMed
-
- Moxley RT III, Ashwal S, Pandya S, et al. . Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2005;64:13–20. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, et al. . Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77–93. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, et al. . Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010;9:177–189. - PubMed
-
- Griggs RC, Moxley RT III, Mendell JR, et al. . Prednisone in Duchenne dystrophy: a randomized, controlled trial defining the time course and dose response: clinical Investigation of Duchenne Dystrophy Group. Arch Neurol 1991;48:383–388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
