A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling
- PMID: 27566765
- PMCID: PMC5161031
- DOI: 10.1158/1078-0432.CCR-16-0190
A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling
Abstract
Purpose: To assess the safety and tolerability of preoperative cryoablation-mediated tumor antigen presentation and/or ipilimumab-mediated immune modulation in women with operable breast cancer.
Experimental design: In this pilot study, 19 women with breast cancer for whom mastectomy was planned were treated with preoperative tumor cryoablation (n = 7), single-dose ipilimumab at 10 mg/kg (n = 6), or both (n = 6). The primary outcome for this pilot study was safety/tolerability as defined as freedom from delays in pre-planned, curative-intent mastectomy. Exploratory studies of immune activation were performed on peripheral blood and tumor.
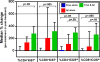
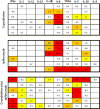
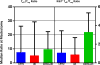
Results: Preoperative cryoablation and/or ipilimumab were safe and tolerable, with no delays in pre-planned surgery. Grade III toxicity was seen in 1 of 19 (unrelated rash after ipilimumab). Combination therapy was associated with sustained peripheral elevations in: Th1-type cytokines, activated (ICOS+) and proliferating (Ki67+) CD4+ and CD8+ T cells, and posttreatment proliferative T-effector cells relative to T-regulatory cells within tumor.
Conclusions: Preoperative cryoablation and single-dose ipilimumab are safe alone or in combination with no surgical delays incurred. Potentially favorable intratumoral and systemic immunologic effects were observed with the combination, suggesting the possibility for induced and synergistic antitumor immunity with this strategy. Clin Cancer Res; 22(23); 5729-37. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Heather L. McArthur has research supported by Bristol-Myers Squibb (BMS); Jianda Yuan has a commercial grant from BMS; Padmanee Sharma has consulted for BMS, has research supported by BMS, and has a spouse with patents licensed to BMS; James P. Allison has intellectual property and royalties from BMS; and Jedd D. Wolchok has grant support from BMS and has consulted for BMS.
Figures

References
-
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
-
- Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–94. - PubMed
-
- Emens LA. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). AACR Annual Meeting; Philadelphia, PA. April 20, 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

