Understanding and applying pharmacometric modelling and simulation in clinical practice and research
- PMID: 27567102
- PMCID: PMC5237699
- DOI: 10.1111/bcp.13119
Understanding and applying pharmacometric modelling and simulation in clinical practice and research
Abstract
Understanding the dose-concentration-effect relationship is a fundamental component of clinical pharmacology. Interpreting data arising from observations of this relationship requires the use of mathematical models; i.e. pharmacokinetic (PK) models to describe the relationship between dose and concentration and pharmacodynamic (PD) models describing the relationship between concentration and effect. Drug development requires several iterations of pharmacometric model-informed learning and confirming. This includes modelling to understand the dose-response in preclinical studies, deriving a safe dose for first-in-man, and the overall analysis of Phase I/II data to optimise the dose for safety and efficacy in Phase III pivotal trials. However, drug development is not the boundary at which PKPD understanding and application stops. PKPD concepts will be useful to anyone involved in the prescribing and administration of medicines for purposes such as determining off-label dosing in special populations, individualising dosing based on a measured biomarker (personalised medicine) and in determining whether lack of efficacy or unexpected toxicity maybe solved by adjusting the dose rather than the drug. In clinical investigator-led study design, PKPD can be used to ensure the optimal dose is used, and crucially to define the expected effect size, thereby ensuring power calculations are based on sound prior information. In the clinical setting the most likely people to hold sufficient expertise to advise on PKPD matters will be the pharmacists and clinical pharmacologists. This paper reviews fundamental PKPD principles and provides some real-world examples of PKPD use in clinical practice and applied clinical research.
Keywords: pharmacodynamics; pharmacokinetic-pharmacodynamic modelling; pharmacokinetics.
© 2016 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
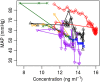
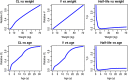
References
-
- Almond CS, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med 2005; 352: 1550–1556. - PubMed
-
- Barker CI, Standing JF, Turner MA, McElnay JC, Sharland M. Antibiotic dosing in children in Europe: can we grade the evidence from pharmacokinetic/pharmacodynamic studies ‐ and when is enough data enough? Curr Opin Infect Dis 2012; 25: 235–242. - PubMed
-
- Standing JF, Hammer GB, Sam WJ, Drover DR. Pharmacokinetic–pharmacodynamic modeling of the hypotensive effect of remifentanil in infants undergoing cranioplasty. Paediatr Anaesth 2010; 20: 7–18. - PubMed
-
- Ette EI, Williams PJ. Population pharmacokinetics I: background, concepts, and models. Ann Pharmacother 2004; 38: 1702–1706. - PubMed
-
- Ette EI, Williams PJ. Population pharmacokinetics II: estimation methods. Ann Pharmacother 2004; 38: 1907–1915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

