Sociability Deficits and Altered Amygdala Circuits in Mice Lacking Pcdh10, an Autism Associated Gene
- PMID: 27567313
- PMCID: PMC5161717
- DOI: 10.1016/j.biopsych.2016.06.008
Sociability Deficits and Altered Amygdala Circuits in Mice Lacking Pcdh10, an Autism Associated Gene
Abstract
Background: Behavioral symptoms in individuals with autism spectrum disorder (ASD) have been attributed to abnormal neuronal connectivity, but the molecular bases of these behavioral and brain phenotypes are largely unknown. Human genetic studies have implicated PCDH10, a member of the δ2 subfamily of nonclustered protocadherin genes, in ASD. PCDH10 expression is enriched in the basolateral amygdala, a brain region implicated in the social deficits of ASD. Previous reports indicate that Pcdh10 plays a role in axon outgrowth and glutamatergic synapse elimination, but its roles in social behaviors and amygdala neuronal connectivity are unknown. We hypothesized that haploinsufficiency of Pcdh10 would reduce social approach behavior and alter the structure and function of amygdala circuits.
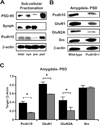
Methods: Mice lacking one copy of Pcdh10 (Pcdh10+/-) and wild-type littermates were assessed for social approach and other behaviors. The lateral/basolateral amygdala was assessed for dendritic spine number and morphology, and amygdala circuit function was studied using voltage-sensitive dye imaging. Expression of Pcdh10 and N-methyl-D-aspartate receptor (NMDAR) subunits was assessed in postsynaptic density fractions of the amygdala.
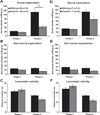
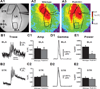
Results: Male Pcdh10+/- mice have reduced social approach behavior, as well as impaired gamma synchronization, abnormal spine morphology, and reduced levels of NMDAR subunits in the amygdala. Social approach deficits in Pcdh10+/- male mice were rescued with acute treatment with the NMDAR partial agonist d-cycloserine.
Conclusions: Our studies reveal that male Pcdh10+/- mice have synaptic and behavioral deficits, and establish Pcdh10+/- mice as a novel genetic model for investigating neural circuitry and behavioral changes relevant to ASD.
Keywords: Amygdala; Autism; Gene; NMDA; Protocadherin; Synapse.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures Warren Bilker has consulted for Janssen Pharmaceuticals. The consulting is not related to the subject matter of the manuscript. Robert T. Schultz has received consulting fees from Akili Inc, Johnson & Johnson Consumer Inc, and Lumos Pharma Inc. The consulting is not related to the subject matter of the manuscript. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures
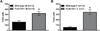


Comment in
-
Protocadherins and the Social Brain.Biol Psychiatry. 2017 Feb 1;81(3):173-174. doi: 10.1016/j.biopsych.2016.10.025. Biol Psychiatry. 2017. PMID: 28024704 Free PMC article. No abstract available.
References
-
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet. 2001;98(2):161–167. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

