Automated identification of an aspirin-exacerbated respiratory disease cohort
- PMID: 27567328
- PMCID: PMC5266739
- DOI: 10.1016/j.jaci.2016.05.048
Automated identification of an aspirin-exacerbated respiratory disease cohort
Abstract
Background: Aspirin-exacerbated respiratory disease (AERD) is characterized by 3 clinical features: asthma, nasal polyposis, and respiratory reactions to cyclooxygenase-1 inhibitors (nonsteroidal anti-inflammatory drugs). Electronic health records (EHRs) contain information on each feature of this triad.
Objective: We sought to determine whether an informatics algorithm applied to the EHR could electronically identify patients with AERD.
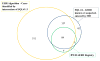
Methods: We developed an informatics algorithm to search the EHRs of patients aged 18 years and older from the Partners Healthcare system over a 10-year period (2004-2014). Charts with search terms for asthma, nasal polyps, and record of respiratory (cohort A) or unspecified (cohort B) reactions to nonsteroidal anti-inflammatory drugs were identified as "possible AERD." Two clinical experts reviewed all charts to confirm a diagnosis of "clinical AERD" and classify cases as "diagnosed AERD" or "undiagnosed AERD" on the basis of physician-documented AERD-specific terms in patient notes.
Results: Our algorithm identified 731 "possible AERD" cases, of which 638 were not in our AERD patient registry. Chart review of cohorts A (n = 511) and B (n = 127) demonstrated a positive predictive value of 78.4% for "clinical AERD," which rose to 88.7% when unspecified reactions were excluded. Of those with clinical AERD, 12.4% had no mention of AERD by any treating caregiver and were classified as "undiagnosed AERD." "Undiagnosed AERD" cases were less likely than "diagnosed AERD" cases to have been seen by an allergist/immunologist (38.7% vs 93.2%; P < .0001).
Conclusions: An informatics algorithm can successfully identify both known and previously undiagnosed cases of AERD with a high positive predictive value. Involvement of an allergist/immunologist significantly increases the likelihood of an AERD diagnosis.
Keywords: Aspirin-exacerbated respiratory disease; asthma; chronic rhinosinusitis; clinical decision support; electronic health record; nasal polyps; nonsteroidal anti-inflammatory drugs; structured query language.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. The Journal of allergy and clinical immunology. 2015;135(3):676–81. e1. - PubMed
-
- Kowalski ML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, Bochenek G, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013;68(10):1219–32. - PubMed
-
- Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. The Journal of allergy and clinical immunology. 2014;133(1):98–103. e1–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

