A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report
- PMID: 27567635
- PMCID: PMC5566491
- DOI: 10.1007/s10143-016-0781-0
A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report
Erratum in
-
Erratum to: A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report.Neurosurg Rev. 2017 Jan;40(1):177. doi: 10.1007/s10143-016-0787-7. Neurosurg Rev. 2017. PMID: 27718100 Free PMC article. No abstract available.
Abstract
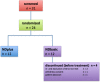
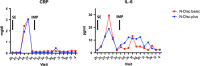
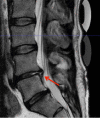
NOVOCART® Disk plus, an autologous cell compound for autologous disk chondrocyte transplantation, was developed to reduce the degenerative sequel after lumbar disk surgery or to prophylactically avoid degeneration in adjacent disks, if present. The NDisc trial is an ongoing multi-center, randomized study with a sequential phase I study within the combined phase I/II trial with close monitoring of tolerability and safety. Twenty-four adult patients were randomized and treated with the investigational medicinal product NDisc plus or the carrier material only. Rates of adverse events in Phase I of this trial were comparable with those expected in the early time course after elective disk surgery. There was one reherniation 7 months after transplantation, which corresponds to an expected reherniation rate. Immunological markers like CRP and IL-6 were not significantly elevated and there were no imaging abnormalities. No indications of harmful material extrusion or immunological consequences due to the investigational medicinal product NDplus were observed. Therefore, the study appears to be safe and feasible. Safety analyses of Phase I of this trial indicate a relatively low risk considering the benefits that patients with debilitating degenerative disk disease may gain.
Keywords: Autologous disk chondrocyte transplantation; Degenerative disk disease; Lumbar back pain; Sequestrectomy.
Conflict of interest statement
TETEC AG, a shared company of B|Braun Aesculap, Tuttlingen, Germany sponsors this study and this publication.
The departments received grant support to perform the study. Steinert and Michnacs are employee of TETEC AG.
Figures
References
-
- Borenstein DG, O'Mara JW, Boden SD, Lauerman WC, Jacobson A, Platenberg C, et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects : a seven-year follow-up study. J Bone Joint Surg Am. 2001;83-A:1306–1311. doi: 10.2106/00004623-200109000-00002. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

