Clinical Application of Automatic Segmentation of Medial Temporal Lobe Subregions in Prodromal and Dementia-Level Alzheimer's Disease
- PMID: 27567809
- PMCID: PMC5520993
- DOI: 10.3233/JAD-160014
Clinical Application of Automatic Segmentation of Medial Temporal Lobe Subregions in Prodromal and Dementia-Level Alzheimer's Disease
Abstract
Background: Volumetry of medial temporal lobe (MTL) structures to diagnose Alzheimer's disease (AD) in its earliest symptomatic stage could be of great importance for interventions or disease modifying pharmacotherapy.
Objective: This study aimed to demonstrate the first application of an automatic segmentation method of MTL subregions in a clinical population. Automatic segmentation of magnetic resonance images (MRIs) in a research population has previously been shown to detect evidence of neurodegeneration in MTL subregions and to help discriminate AD and mild cognitive impairment (MCI) from a healthy comparison group.
Methods: Clinical patients were selected and T2-weighted MRI scan quality was checked. An automatic segmentation method of hippocampal subfields (ASHS) was applied to scans of 67 AD patients, 38 amnestic MCI patients, and 57 healthy controls. Hippocampal subfields, entorhinal cortex (ERC), and perirhinal cortex were automatically labeled and subregion volumes were compared between groups.
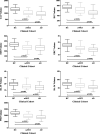
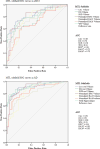
Results: One fourth of all scans were excluded due to bad scan quality. There were significant volume reductions in all subregions, except BA36, in aMCIs (p < 0.001), most prominently in Cornu Ammonis 1 (CA1) and ERC, and in all subregions in AD. However, sensitivity of CA1 and ERC hardly differed from sensitivity of WH in aMCI and AD.
Conclusion: Applying automatic segmentation of MTL subregions in a clinical setting as a potential biomarker for prodromal AD is feasible, but issues of image quality due to motion remain to be addressed. CA1 and ERC provided strongest group discrimination in differentiating aMCIs from controls, but discriminatory power of different subfields was low overall.
Keywords: Alzheimer’s disease; Cornu Ammonis; anatomy; biomarker; diagnosis; entorhinal cortex; hippocampal subfields; hippocampus; histology; magnetic resonance imaging; medial temporal lobe; mild cognitive impairment.
Figures

Similar articles
-
Automated segmentation of medial temporal lobe subregions on in vivo T1-weighted MRI in early stages of Alzheimer's disease.Hum Brain Mapp. 2019 Aug 15;40(12):3431-3451. doi: 10.1002/hbm.24607. Epub 2019 Apr 29. Hum Brain Mapp. 2019. PMID: 31034738 Free PMC article.
-
The Effect of Segmentation Method on Medial Temporal Lobe Subregion Volumes in Aging.Hum Brain Mapp. 2024 Oct 15;45(15):e70054. doi: 10.1002/hbm.70054. Hum Brain Mapp. 2024. PMID: 39450487 Free PMC article.
-
Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment.Hum Brain Mapp. 2015 Jan;36(1):258-87. doi: 10.1002/hbm.22627. Epub 2014 Sep 2. Hum Brain Mapp. 2015. PMID: 25181316 Free PMC article.
-
Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment.Cochrane Database Syst Rev. 2020 Mar 2;3(3):CD009628. doi: 10.1002/14651858.CD009628.pub2. Cochrane Database Syst Rev. 2020. PMID: 32119112 Free PMC article.
-
Morphometry of medial temporal lobe subregions using high-resolution T2-weighted MRI in ADNI3: Why, how, and what's next?Alzheimers Dement. 2024 Nov;20(11):8113-8128. doi: 10.1002/alz.14161. Epub 2024 Sep 16. Alzheimers Dement. 2024. PMID: 39279366 Free PMC article. Review.
Cited by
-
Recognition Memory Dysfunction Relates to Hippocampal Subfield Volume: A Study of Cognitively Normal and Mildly Impaired Older Adults.J Gerontol B Psychol Sci Soc Sci. 2019 Sep 15;74(7):1132-1141. doi: 10.1093/geronb/gbx181. J Gerontol B Psychol Sci Soc Sci. 2019. PMID: 29401233 Free PMC article.
-
Do multiple system atrophy and Parkinson's disease show distinct patterns of volumetric alterations across hippocampal subfields? An exploratory study.Eur Radiol. 2019 Sep;29(9):4948-4956. doi: 10.1007/s00330-019-06043-9. Epub 2019 Feb 22. Eur Radiol. 2019. PMID: 30796577
-
Diagnostic performance of hippocampal volumetry in Alzheimer's disease or mild cognitive impairment: a meta-analysis.Eur Radiol. 2022 Oct;32(10):6979-6991. doi: 10.1007/s00330-022-08838-9. Epub 2022 May 4. Eur Radiol. 2022. PMID: 35507052
-
The Key Role of Magnetic Resonance Imaging in the Detection of Neurodegenerative Diseases-Associated Biomarkers: A Review.Mol Neurobiol. 2022 Oct;59(10):5935-5954. doi: 10.1007/s12035-022-02944-x. Epub 2022 Jul 12. Mol Neurobiol. 2022. PMID: 35829831 Review.
-
Association between subfield volumes of the medial temporal lobe and cognitive assessments.Heliyon. 2019 Jun 4;5(6):e01828. doi: 10.1016/j.heliyon.2019.e01828. eCollection 2019 Jun. Heliyon. 2019. PMID: 31194147 Free PMC article.
References
-
- Snyder PJ, Kahle-Wrobleski K, Brannan S, Miller DS, Schindler RJ, DeSanti S, Ryan JM, Morrison G, Grundman M, Chandler J, Caselli RJ, Isaac M, Bain L, Carrillo MC. Assessing cognition and function in Alzheimer’s disease clinical trials: Do we have the right tools? Alzheimers Dement. 2014;10:853–860. - PubMed
-
- Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, van Berckel BN, Seeley WW, Pijnenburg YA, Gorno-Tempini ML, Kramer JH, Barkhof F, Rosen HJ, van der Flier WM, Jagust WJ, Miller BL, Scheltens P, Rabinovici GD. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36:4421–4437. - PMC - PubMed
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. - PMC - PubMed
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

