A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer's Disease
- PMID: 27567810
- PMCID: PMC5026133
- DOI: 10.3233/JAD-160017
A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer's Disease
Abstract
Background: Deep brain stimulation (DBS) is used to modulate the activity of dysfunctional brain circuits. The safety and efficacy of DBS in dementia is unknown.
Objective: To assess DBS of memory circuits as a treatment for patients with mild Alzheimer's disease (AD).
Methods: We evaluated active "on" versus sham "off" bilateral DBS directed at the fornix-a major fiber bundle in the brain's memory circuit-in a randomized, double-blind trial (ClinicalTrials.gov NCT01608061) in 42 patients with mild AD. We measured cognitive function and cerebral glucose metabolism up to 12 months post-implantation.
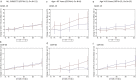
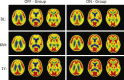
Results: Surgery and electrical stimulation were safe and well tolerated. There were no significant differences in the primary cognitive outcomes (ADAS-Cog 13, CDR-SB) in the "on" versus "off" stimulation group at 12 months for the whole cohort. Patients receiving stimulation showed increased metabolism at 6 months but this was not significant at 12 months. On post-hoc analysis, there was a significant interaction between age and treatment outcome: in contrast to patients <65 years old (n = 12) whose results trended toward being worse with DBS ON versus OFF, in patients≥65 (n = 30) DBS-f ON treatment was associated with a trend toward both benefit on clinical outcomes and a greater increase in cerebral glucose metabolism.
Conclusion: DBS for AD was safe and associated with increased cerebral glucose metabolism. There were no differences in cognitive outcomes for participants as a whole, but participants aged≥65 years may have derived benefit while there was possible worsening in patients below age 65 years with stimulation.
Keywords: Keywords: Alzheimer’s disease; deep brain stimulation; dementia; fornix.
Figures
Similar articles
-
Bilateral deep brain stimulation of the fornix for Alzheimer's disease: surgical safety in the ADvance trial.J Neurosurg. 2016 Jul;125(1):75-84. doi: 10.3171/2015.6.JNS15716. Epub 2015 Dec 18. J Neurosurg. 2016. PMID: 26684775 Free PMC article. Clinical Trial.
-
Deep Brain Stimulation Targeting the Fornix for Mild Alzheimer Dementia (the ADvance Trial): A Two Year Follow-up Including Results of Delayed Activation.J Alzheimers Dis. 2018;64(2):597-606. doi: 10.3233/JAD-180121. J Alzheimers Dis. 2018. PMID: 29914028 Free PMC article.
-
Efficacy and Safety of Bilateral Deep Brain Stimulation (DBS) for Severe Alzheimer's Disease: A Comparative Analysis of Fornix Versus Basal Ganglia of Meynert.CNS Neurosci Ther. 2025 Apr;31(4):e70285. doi: 10.1111/cns.70285. CNS Neurosci Ther. 2025. PMID: 40243219 Free PMC article.
-
Deep brain stimulation of fornix in Alzheimer's disease: From basic research to clinical practice.Eur J Clin Invest. 2023 Aug;53(8):e13995. doi: 10.1111/eci.13995. Epub 2023 Apr 10. Eur J Clin Invest. 2023. PMID: 37004153 Review.
-
Deep brain stimulation for the treatment of Alzheimer disease and dementias.World Neurosurg. 2013 Sep-Oct;80(3-4):S28.e1-8. doi: 10.1016/j.wneu.2012.06.028. Epub 2012 Jun 19. World Neurosurg. 2013. PMID: 22722036 Review.
Cited by
-
CVN-AD Alzheimer's mice show premature reduction in neurovascular coupling in response to spreading depression and anoxia compared to aged controls.Alzheimers Dement. 2021 Jul;17(7):1109-1120. doi: 10.1002/alz.12289. Epub 2021 Mar 3. Alzheimers Dement. 2021. PMID: 33656270 Free PMC article.
-
Brain Stimulation in Alzheimer's Disease.J Alzheimers Dis. 2016 Sep 6;54(2):789-91. doi: 10.3233/JAD-160719. J Alzheimers Dis. 2016. PMID: 27567879 Free PMC article.
-
Functional and anatomical connectivity predict brain stimulation's mnemonic effects.Cereb Cortex. 2024 Jan 14;34(1):bhad427. doi: 10.1093/cercor/bhad427. Cereb Cortex. 2024. PMID: 38041253 Free PMC article.
-
Forniceal deep brain stimulation in a mouse model of Rett syndrome increases neurogenesis and hippocampal memory beyond the treatment period.Brain Stimul. 2023 Sep-Oct;16(5):1401-1411. doi: 10.1016/j.brs.2023.09.002. Epub 2023 Sep 11. Brain Stimul. 2023. PMID: 37704033 Free PMC article.
-
The effects of direct brain stimulation in humans depend on frequency, amplitude, and white-matter proximity.Brain Stimul. 2020 Sep-Oct;13(5):1183-1195. doi: 10.1016/j.brs.2020.05.009. Epub 2020 May 21. Brain Stimul. 2020. PMID: 32446925 Free PMC article.
References
-
- Smith GS, de Leon MJ, George AE, Kluger A, Volkow ND, McRae T, Golomb J, Ferris SH, Reisberg B, Ciaravino J, La Regina ME (1992) Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer’s disease. Pathophysiologic implications. Arch Neurol 49, 1142–1150. - PubMed
-
- Zhou J, Seeley WW (2014) Network dysfunction in Alzheimer’s disease and frontotemporal dementia: Implications for psychiatry. Biol Psychiatry 75, 565–573. - PubMed
-
- Jacobs HI, Radua J, Luckmann HC, Sack AT (2013) Meta-analysis of functional network alterations in Alzheimer’s disease: Toward a network biomarker. Neurosci Biobehav Rev 37, 753–765. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

