Enthalpy screen of drug candidates
- PMID: 27567994
- PMCID: PMC5035635
- DOI: 10.1016/j.ab.2016.08.023
Enthalpy screen of drug candidates
Abstract
The enthalpic and entropic contributions to the binding affinity of drug candidates have been acknowledged to be important determinants of the quality of a drug molecule. These quantities, usually summarized in the thermodynamic signature, provide a rapid assessment of the forces that drive the binding of a ligand. Having access to the thermodynamic signature in the early stages of the drug discovery process will provide critical information towards the selection of the best drug candidates for development. In this paper, the Enthalpy Screen technique is presented. The enthalpy screen allows fast and accurate determination of the binding enthalpy for hundreds of ligands. As such, it appears to be ideally suited to aid in the ranking of the hundreds of hits that are usually identified after standard high throughput screening.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

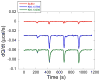
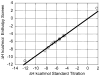
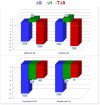
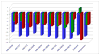
References
-
- Ohtaka H, Freire E. Adaptive inhibitors of the HIV-1 protease. Prog Biophys Mol Biol. 2005;88:193–208. - PubMed
-
- Ladbury JE, Klebe G, Freire E. Adding calorimetric data to decision making in lead discovery: a hot tip. Nat Rev Drug Discov. 2010;9:23–27. - PubMed
-
- Carbonell T, Freire E. Binding thermodynamics of statins to HMG-CoA reductase. Biochemistry. 2005;44:11741–11748. - PubMed
-
- Sarver RW, Peevers J, Cody WL, Ciske FL, Dyer J, Emerson SD, Hagadorn JC, Holsworth DD, Jalaie M, Kaufman M, Mastronardi M, McConnell P, Powell NA, Quin J, 3rd, Van Huis CA, Zhang E, Mochalkin I. Binding thermodynamics of substituted diaminopyrimidine renin inhibitors. Anal Biochem. 2007;360:30–40. - PubMed
-
- Chaires JB. Calorimetry and thermodynamics in drug design. Annu Rev Biophys. 2008;37:135–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

