Indole-based allosteric inhibitors of HIV-1 integrase
- PMID: 27568085
- PMCID: PMC5018460
- DOI: 10.1016/j.bmcl.2016.08.037
Indole-based allosteric inhibitors of HIV-1 integrase
Abstract
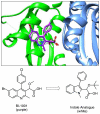
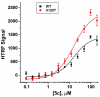
Employing a scaffold hopping approach, a series of allosteric HIV-1 integrase (IN) inhibitors (ALLINIs) have been synthesized based on an indole scaffold. These compounds incorporate the key elements utilized in quinoline-based ALLINIs for binding to the IN dimer interface at the principal LEDGF/p75 binding pocket. The most potent of these compounds displayed good activity in the LEDGF/p75 dependent integration assay (IC50=4.5μM) and, as predicted based on the geometry of the five- versus six-membered ring, retained activity against the A128T IN mutant that confers resistance to many quinoline-based ALLINIs.
Keywords: ALLINI; HIV integrase inhibitor; Scaffold hopping.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures




References
-
- Summa V, Petrocchi A, Bonelli F, Crescenzi B, Donghi M, Ferrara M, Fiore F, Gardelli C, Paz OG, Hazuda DJ, Jones P, Kinzel O, Laufer R, Monteagudo E, Muraglia E, Nizi E, Orvieto F, Pace P, Pescatore G, Scarpelli R, Stillmock K, Witmer MV, Rowley M. J. Med. Chem. 2008;51:5843. - PubMed
-
- Bailly F, Cotelle P. Expert Opin. Drug Discov. 2015;10:1243. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

