In vivo definition of cardiac myosin-binding protein C's critical interactions with myosin
- PMID: 27568194
- PMCID: PMC5308206
- DOI: 10.1007/s00424-016-1873-y
In vivo definition of cardiac myosin-binding protein C's critical interactions with myosin
Abstract
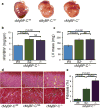
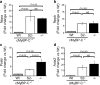
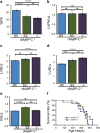
Cardiac myosin-binding protein C (cMyBP-C) is an integral part of the sarcomeric machinery in cardiac muscle that enables normal function. cMyBP-C regulates normal cardiac contraction by functioning as a brake through interactions with the sarcomere's thick, thin, and titin filaments. cMyBP-C's precise effects as it binds to the different filament systems remain obscure, particularly as it impacts on the myosin heavy chain's head domain, contained within the subfragment 2 (S2) region. This portion of the myosin heavy chain also contains the ATPase activity critical for myosin's function. Mutations in myosin's head, as well as in cMyBP-C, are a frequent cause of familial hypertrophic cardiomyopathy (FHC). We generated transgenic lines in which endogenous cMyBP-C was replaced by protein lacking the residues necessary for binding to S2 (cMyBP-C(S2-)). We found, surprisingly, that cMyBP-C lacking the S2 binding site is incorporated normally into the sarcomere, although systolic function is compromised. We show for the first time the acute and chronic in vivo consequences of ablating a filament-specific interaction of cMyBP-C. This work probes the functional consequences, in the whole animal, of modifying a critical structure-function relationship, the protein's ability to bind to a region of the critical enzyme responsible for muscle contraction, the subfragment 2 domain of the myosin heavy chain. We show that the binding is not critical for the protein's correct insertion into the sarcomere's architecture, but is essential for long-term, normal function in the physiological context of the heart.
Keywords: Cardiac; Heart; Myofilament; Myosin; Myosin-binding protein C; Sarcomere.
Figures



References
-
- Carrier L, Bonne G, Bahrend E, Yu B, Richard P, Niel F, Hainque B, Cruaud C, Gary F, Labeit S, Bouhour JB, Dubourg O, Desnos M, Hagege AA, Trent RJ, Komajda M, Fiszman M, Schwartz K. Organization and sequence of human cardiac myosin binding protein C gene (MYBPC3) and identification of mutations predicted to produce truncated proteins in familial hypertrophic cardiomyopathy. Circ Res. 1997;80:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

