Fine motor control of the jaw following alteration of orofacial afferent inputs
- PMID: 27568306
- PMCID: PMC5318472
- DOI: 10.1007/s00784-016-1939-4
Fine motor control of the jaw following alteration of orofacial afferent inputs
Abstract
Objective: The study was designed to investigate if alteration of different orofacial afferent inputs would have different effects on oral fine motor control and to test the hypothesis that reduced afferent inputs will increase the variability of bite force values and jaw muscle activity, and repeated training with splitting of food morsel in conditions with reduced afferent inputs would decrease the variability and lead to optimization of bite force values and jaw muscle activity.
Material methods: Forty-five healthy volunteers participated in a single experimental session and were equally divided into incisal, mucosal, and block anesthesia groups. The participants performed six series (with ten trials) of a standardized hold and split task after the intervention with local anesthesia was made in the respective groups. The hold and split forces along with the corresponding jaw muscle activity were recorded and compared to a reference group.
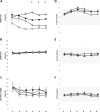
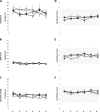
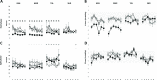
Results: The hold force and the electromyographic (EMG) activity of the masseter muscles during the hold phase were significantly higher in the incisal and block anesthesia group, as compared to the reference group (P < 0.001). However, there was no significant effect of groups on the split force (P = 0.975) but a significant decrease in the EMG activity of right masseter in mucosal anesthesia group as compared to the reference group (P = 0.006). The results also revealed that there was no significant effect of local anesthesia on the variability of the hold and split force (P < 0.677). However, there was a significant decrease in the variability of EMG activity of the jaw closing muscles in the block anesthesia group as compared to the reference group (P < 0.041), during the hold phase and a significant increase in the variability of EMG activity of right masseter in the mucosal anesthesia group (P = 0.021) along with a significant increase in the EMG activity of anterior temporalis muscle in the incisal anesthesia group, compared to the reference group (P = 0.018), during the split phase.
Conclusions: The results of the present study indicated that altering different orofacial afferent inputs may have different effects on some aspects of oral fine motor control. Further, inhibition of afferent inputs from the orofacial or periodontal mechanoreceptors did not increase the variability of bite force values and jaw muscle activity; indicating that the relative precision of the oral fine motor task was not compromised inspite of the anesthesia. The results also suggest the propensity of optimization of bite force values and jaw muscle activity due to repeated splitting of the food morsels, inspite of alteration of sensory inputs.
Clinical relevance: Skill acquisition following a change in oral sensory environment is crucial for understanding how humans learn and re-learn oral motor behaviors and the kind of adaptation that takes place after successful oral rehabilitation procedures.
Keywords: Bite force; Local anesthesia; Optimization; Periodontal mechanoreceptors; Variability.
Conflict of interest statement
Conflict of interest
Author Abhishek Kumar declares that he has no conflict of interest. Author Eduardo Castrillon declares that he has no conflict of interest. Author Krister G. Svensson declares that he has no conflict of interest. Author Mats Trulsson declares that he has no conflict of interest. Author Peter Svensson declares that he has no conflict of interest.
Funding
The work was supported by the Section of Orofacial Pain and Jaw Function, Department of Dentistry, Aarhus University, and the Danish Dental Association, Denmark.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki II and approved by the ethics committee, Midjutland region, Denmark.
Informed consent
Informed consent was obtained from all the participants prior to the start of the experiment.
Figures


Similar articles
-
Optimization of jaw muscle activity and fine motor control during repeated biting tasks.Arch Oral Biol. 2014 Dec;59(12):1342-51. doi: 10.1016/j.archoralbio.2014.08.009. Epub 2014 Aug 23. Arch Oral Biol. 2014. PMID: 25193315
-
Effects of experimental craniofacial pain on fine jaw motor control: a placebo-controlled double-blinded study.Exp Brain Res. 2015 Jun;233(6):1745-59. doi: 10.1007/s00221-015-4245-5. Epub 2015 Mar 19. Exp Brain Res. 2015. PMID: 25788006 Clinical Trial.
-
Task-dependent control of the jaw during food splitting in humans.J Neurophysiol. 2014 Jun 15;111(12):2614-23. doi: 10.1152/jn.00797.2013. Epub 2014 Mar 26. J Neurophysiol. 2014. PMID: 24671539
-
Electromyography of the anterior temporalis and masseter muscles of owl monkeys (Aotus trivirgatus) and the function of the postorbital septum.Am J Phys Anthropol. 2000 Aug;112(4):455-68. doi: 10.1002/1096-8644(200008)112:4<455::AID-AJPA4>3.0.CO;2-4. Am J Phys Anthropol. 2000. PMID: 10918124 Review.
-
Symphyseal fusion and jaw-adductor muscle force: an EMG study.Am J Phys Anthropol. 2000 Aug;112(4):469-92. doi: 10.1002/1096-8644(200008)112:4<469::AID-AJPA5>3.0.CO;2-V. Am J Phys Anthropol. 2000. PMID: 10918125 Review.
Cited by
-
Motor Performance and Skill Acquisition in Oral Motor Training With Exergames: A Pilot Study.Front Aging Neurosci. 2022 Mar 2;14:730072. doi: 10.3389/fnagi.2022.730072. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35309890 Free PMC article.
-
Effect of Sudden Deprivation of Sensory Inputs From Periodontium on Mastication.Front Neurosci. 2019 Dec 10;13:1316. doi: 10.3389/fnins.2019.01316. eCollection 2019. Front Neurosci. 2019. PMID: 31920486 Free PMC article.
-
Effects of Chronic and Experimental Acute Masseter Pain on Precision Biting Behavior in Humans.Front Physiol. 2019 Oct 29;10:1369. doi: 10.3389/fphys.2019.01369. eCollection 2019. Front Physiol. 2019. PMID: 31736787 Free PMC article.
-
Training-induced dynamics of accuracy and precision in human motor control.Sci Rep. 2017 Jul 28;7(1):6784. doi: 10.1038/s41598-017-07078-y. Sci Rep. 2017. PMID: 28754929 Free PMC article.
-
Tactile Sensibility Thresholds in Implant Prosthesis, Complete Dentures and Natural Dentition: Review about Their Value in Literature.Medicina (Kaunas). 2022 Mar 31;58(4):501. doi: 10.3390/medicina58040501. Medicina (Kaunas). 2022. PMID: 35454340 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

