NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone
- PMID: 27568565
- PMCID: PMC5014649
- DOI: 10.1016/j.celrep.2016.08.002
NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone
Abstract
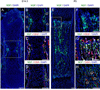
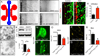
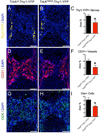
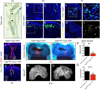
Developing tissues dictate the amount and type of innervation they require by secreting neurotrophins, which promote neuronal survival by activating distinct tyrosine kinase receptors. Here, we show that nerve growth factor (NGF) signaling through neurotrophic tyrosine kinase receptor type 1 (TrkA) directs innervation of the developing mouse femur to promote vascularization and osteoprogenitor lineage progression. At the start of primary ossification, TrkA-positive axons were observed at perichondrial bone surfaces, coincident with NGF expression in cells adjacent to centers of incipient ossification. Inactivation of TrkA signaling during embryogenesis in TrkA(F592A) mice impaired innervation, delayed vascular invasion of the primary and secondary ossification centers, decreased numbers of Osx-expressing osteoprogenitors, and decreased femoral length and volume. These same phenotypic abnormalities were observed in mice following tamoxifen-induced disruption of NGF in Col2-expressing perichondrial osteochondral progenitors. We conclude that NGF serves as a skeletal neurotrophin to promote sensory innervation of developing long bones, a process critical for normal primary and secondary ossification.
Keywords: endochondral bone; nerve growth factor; neurotrophic tyrosine kinase receptor type 1; sensory nerves.
Published by Elsevier Inc.
Figures



References
-
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. - PubMed
-
- Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–171. - PubMed
-
- Bonkowsky JL, Johnson J, Carey JC, Smith AG, Swoboda KJ. An infant with primary tooth loss and palmar hyperkeratosis: a novel mutation in the NTRK1 gene causing congenital insensitivity to pain with anhidrosis. Pediatrics. 2003;112:e237–e241. - PubMed
-
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

