Effect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States
- PMID: 27568580
- PMCID: PMC6026224
- DOI: 10.1016/j.chest.2016.06.046
Effect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States
Abstract
Background: There is a growing use of procalcitonin (PCT) to facilitate the diagnosis and management of severe sepsis. We investigated the impact of one to two PCT determinations on ICU day 1 on health-care utilization and cost in a large research database.
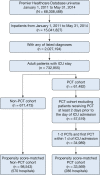
Methods: A retrospective, propensity score-matched multivariable analysis was performed on the Premier Healthcare Database for patients admitted to the ICU with one to two PCT evaluations on day 1 of ICU admission vs patients who did not have PCT testing.
Results: A total of 33,569 PCT-managed patients were compared with 98,543 propensity score-matched non-PCT patients. In multivariable regression analysis, PCT utilization was associated with significantly decreased total length of stay (11.6 days [95% CI, 11.4 to 11.7] vs 12.7 days [95% CI, 12.6 to 12.8]; 95% CI for difference, 1 to 1.3; P < .001) and ICU length of stay (5.1 days [95% CI, 5.1 to 5.2] vs 5.3 days [95% CI, 5.3 to 5.4]; 95% CI for difference, 0.1 to 0.3; P < .03), and lower hospital costs ($30,454 [95% CI, 29,968 to 31,033] vs $33,213 [95% CI, 32,964 to 33,556); 95% CI for difference, 2,159 to 3,321; P < .001). There was significantly less total antibiotic exposure (16.2 days [95% CI, 16.1 to 16.5] vs 16.9 days [95% CI, 16.8 to 17.1]; 95% CI for difference, -0.9 to 0.4; P = .006) in PCT-managed patients. Patients in the PCT group were more likely to be discharged to home (44.1% [95% CI, 43.7 to 44.6] vs 41.3% [95% CI, 41 to 41.6]; 95% CI for difference, 2.3 to 3.3; P = .006). Mortality was not different in an analysis including the 96% of patients who had an independent measure of mortality risk available (19.1% [95% CI, 18.7 to 19.4] vs 19.1% [95% CI, 18.9 to 19.3]; 95% CI for difference, -0.5 to 0.4; P = .93).
Conclusions: Use of PCT testing on the first day of ICU admission was associated with significantly lower hospital and ICU lengths of stay, as well as decreased total, ICU, and pharmacy cost of care. Further elucidation of clinical outcomes requires additional data.
Keywords: antibiotic use; cost of care; intensive care unit; procalcitonin; sepsis.
Copyright © 2016 American College of Chest Physicians. All rights reserved.
Figures

Comment in
-
Additional Real-World Evidence Supporting Procalcitonin as an Effective Tool to Improve Antibiotic Management and Cost of the Critically Ill Patient.Chest. 2017 Jan;151(1):6-8. doi: 10.1016/j.chest.2016.07.014. Chest. 2017. PMID: 28065252 No abstract available.
-
Is a Single Initial Procalcitonin Test Sufficient in Septic, Critically Ill Patients to Minimize Antibiotic Use?Chest. 2017 Jul;152(1):218-219. doi: 10.1016/j.chest.2017.03.062. Chest. 2017. PMID: 28693771 No abstract available.
-
Response.Chest. 2017 Jul;152(1):219-220. doi: 10.1016/j.chest.2017.04.180. Chest. 2017. PMID: 28693772 Free PMC article. No abstract available.
-
Implications of Procalcitonin Testing in Critically Ill Patients with Sepsis.Am J Respir Crit Care Med. 2019 Jan 15;199(2):232-234. doi: 10.1164/rccm.201712-2544RR. Am J Respir Crit Care Med. 2019. PMID: 30423258 No abstract available.
References
-
- Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. HCUP Statistical Brief #160. Rockville, MD: Agency for Healthcare Research and Quality; August 2013. - PubMed
-
- Vincent J.L., Rello J., Marshall J. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. - PubMed
-
- Kaukonen K.M., Bailey M., Pilcher D. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638. - PubMed
-
- Kumar A., Roberts D., Wood K.E. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

